Non-muscle-invasive Bladder Cancer
Part 2
Comprehensive Review Article
Prof. Dr. Semir. A. Salim. Al Samarrai
PREDICTING DISEASE RECURRENCE AND PROGRESSION:
- TaT1 tumours
Treatment should take into account a patient’s prognosis. In order to predict the risk of disease recurrence and/ or progression, several prognostic models for specified patient populations have been introduced.
- Scoring models using the WHO 1973 classification system
The 2006 European Organisation for Research and Treatment of Cancer (EORTC) scoring model to be able to predict both the short- and long-term risks of disease recurrence and progression in individual patients, the EORTC Genito-Urinary Cancer Group (GUCG) published a scoring system and risk tables based on the WHO 1973 classification in 2006 [1]. The scoring system is based on the 6 most significant clinical and pathological factors in patients mainly treated by intravesical chemotherapy:
• Number of tumours;
• Tumour diameter;
• Prior recurrence rate;
• T category;
• Concurrent CIS;
• WHO 1973 tumour grade.
Using the 2006 EORTC scoring model, individual probabilities of recurrence and progression at 1 and 5 years may be calculated (https://www.omnicalculator.com/health/eortc-bladder-cancer).
- The model for patients with Ta G1/G2 (WHO 1973) tumours treated with chemotherapy Patients with Ta G1/G2 tumours receiving chemotherapy were stratified into 3 risk groups for recurrence, taking into account the history of recurrences, history of intravesical treatment, tumour grade (WHO 1973), number of tumours and adjuvant chemotherapy [2].
- Club Urologico Español de Tratamiento Oncologico (CUETO) scoring model for BCG-treated patients A model that predicts the risk of recurrence and progression, based on 12 doses of intravesical BCG over a 5 to 6 months period following TURB, has been published by the CUETO (Spanish Urological Oncology Group). It is based on an analysis of 1,062 patients from 4 CUETO trials that compared different intravesical BCG treatments. No immediate post-operative instillation or second TURB was performed in these patients. The scoring system is based on the evaluation of seven prognostic factors:
• gender;
• age;
• prior recurrence status;
• number of tumours;
• T category;
• associated CIS;
• WHO 1973 tumour grade.
Using this model, the calculated risk of recurrence is lower than that obtained by the EORTC tables. For progression, probability is lower only in high-risk patients [3]. The lower risks in the CUETO tables may be attributed to the use of BCG in this study. The prognostic value of the EORTC scoring system has been confirmed by data from the CUETO patients treated with BCG and by long-term follow-up in an independent patient population [4, 5].
- The 2016 EORTC scoring model for patients treated with maintenance BCG
In 1,812 intermediate- and high-risk patients without CIS treated with 1 to 3 years of maintenance BCG, the EORTC found that the prior disease-recurrence rate and number of tumours were the most important prognostic factors for disease recurrence, stage and WHO 1973 grade for disease progression and diseasespecific survival, while age and WHO 1973 grade were the most important prognostic factors for OS. T1 G3 patients did poorly, with 1- and 5-year disease-progression rates of 11.4% and 19.8%, respectively. Using these data, EORTC risk groups and nomograms for BCG-treated patients were developed [6].
- Scoring model using the WHO 2004/2016 and WHO 1973 classification systems
- EAU NMIBC 2021 scoring model
To update the risk of disease progression and create new prognostic factor risk groups using both the WHO 1973 and WHO 2004/2016 classification systems (without central pathology review), individual patient data from 3,401 primary patients treated from 1990 to 2018 were used [7]. Only patients treated with TURB ± intravesical chemotherapy were included, those treated with adjuvant intravesical BCG were excluded because BCG may reduce the risk of disease progression. From the multivariate analysis, tumour stage, WHO 1973 grade, WHO 2004/2016 grade, concomitant CIS, number of tumours, tumour size and age were independent predictors of disease progression [7]. Further prognostic factors
Further prognostic factors have been described in selected patient populations:
• In T1G3 tumours, important prognostic factors were female sex, CIS in the prostatic urethra in men treated with an induction course of BCG, and age, tumour size and concurrent CIS in BCG-treated patients (62% with an induction course only) [8, 9].
• Attention must be given to patients with T1G3 tumours in bladder (pseudo) diverticulum because of the absence of muscle layer in the diverticular wall [10].
• In patients with T1 tumours, the finding of residual T1 disease at second TURB is an unfavourable prognostic factor [11-13].
- In patients with T1G2 tumours treated with TURB, recurrence at 3 months was the most important predictor of progression [14].
• The prognostic value of pathological factors has been discussed elsewhere. More research is needed to determine the role of molecular markers in improving the predictive accuracy of currently available risk tables [4, 15].
• Pre-operative neutrophil-to-lymphocyte ratio may have prognostic value in NMIBC. This data, however, needs further validation [16].
- Carcinoma in situ
Without any treatment, approximately 54% of patients with CIS progress to muscle-invasive disease [17]. There are no reliable prognostic factors, but some studies, however, have reported a worse prognosis in concurrent CIS and T1 tumours compared to primary CIS [18,19], in extended CIS [20] and in CIS in the prostatic urethra [8]. The response to intravesical treatment with BCG or chemotherapy is an important prognostic factor for subsequent progression and death caused by BC [3–5, 14]. Approximately 10 to 20% of complete responders eventually progress to muscle-invasive disease, compared with 66% of non-responders [21, 22].
- Patient stratification into risk groups
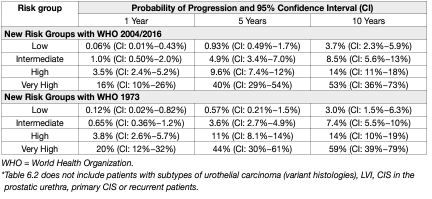
To be able to facilitate treatment recommendations, the Guidelines Panel recommends the stratification of patients into risk groups based on their probability of progression to muscle-invasive disease. The new risk group definitions provided in these EAU Guidelines are based on an IPD analysis in primary patients and the calculation of their progression scores (2021 EAU NMIBC scoring model) [7]. For calculation of the risk group in individual patients, either one, or both, of the WHO 1973 and WHO 2004/2016 classification systems may be used. The probability of progression at 5 years varies from less than 1% to more than 40% between the risk groups. For factors where IPD were not collected such as subtypes of urothelial carcinoma (variant histologies), LVI, primary CIS and CIS in the prostatic urethra; literature data have been used to classify patients into risk groups. The clinical compositions of the new EAU NMIBC prognostic factor risk groups based on the WHO 2004/2016 or WHO 1973 classification systems are provided in Table 1. Apps for the web (www.nmibc.net), iOS and Android (iOS / https://apps.apple.com/us/app/eau-nmibc-risk-calculator/id1578482687 and Android / https://play.google.com/store/apps/details?id=net.ydeal.nmibc) have been developed to facilitate determining a patient’s risk group in daily clinical practice. The individual probability of disease progression at 1, 5 and 10 years for the new EAU NMIBC risk groups is presented in Table 2.
Table 1: Clinical composition of the new EAU NMIBC prognostic factor risk groups based on the WHO 2004/2016 or the WHO 1973 grading classification systems
• Only one of the two classification systems (WHO 1973 or WHO 2004/2016) is required to use this table.
• If both classification systems are available in an individual patient, the EAU Guidelines 2022 edition recommends using the risk group calculation based on the WHO 1973 as it has better prognostic value.
• The category of LG tumours (WHO 2004/2016) also includes patients with tumours classified as PUNLMP.
• Additional clinical risk factors are*:
o age > 70;
o multiple papillary tumours;
o tumour diameter > 3 cm.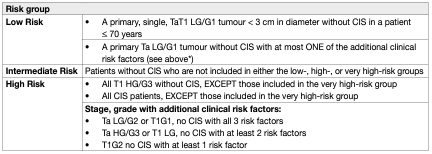

The scoring model is based on individual patient data (IPD), but does not consider patients with primary CIS (high risk) or with recurrent tumours, as well as some pathologic parameters like subtypes of urothelial carcinoma and Lymphovascular invasion (LVI). Nevertheless:
• Based on data from the literature, all patients with CIS in the prostatic urethra, with subtypes of urothelial carcinoma or with LVI should be included in the very high-risk group.
• Patients with recurrent tumours should be included in the intermediate-, high-, or very high-risk groups according to their other prognostic factors.
Table 2: Probabilities of disease progression in 1, 5 and 10 year(s) for the new EAU NMIBC risk groups
DISEASE MANAGEMENT:
- Counselling of smoking cessation
It has been confirmed that smoking increases the risk of tumour recurrence and progression [23, 24]. While it is still controversial whether smoking cessation in BC will favourably influence the outcome of BC treatment, patients should be counselled to stop smoking due to the general risks connected with tobacco smoking [10, 25–27].
- Adjuvant treatment
Although TURB by itself can eradicate a TaT1 tumour completely, these tumours commonly recur and can progress to MIBC. The high variability in the 3-month recurrence rate indicates that the TURB was incomplete or provokes recurrences in a high percentage of patients [28]. It is therefore necessary to consider adjuvant therapy in all patients.
- Intravesical chemotherapy
- A single, immediate, post-operative intravesical instillation of chemotherapy
Immediate single instillation (SI) has been shown to act by destroying circulating tumour cells after TURB, and by an ablative effect on residual tumour cells at the resection site and on small overlooked tumours [29–32]. Four large meta-analyses comprising 1,476 to 3,103 patients have consistently shown that after TURB, SI significantly reduces the recurrence rate compared to TURB alone [33–36]. In a systematic review and IPD meta-analysis of 2,278 eligible patients [33], SI reduced the 5-year recurrence rate by 14%, from 59% to 45%. Only patients with primary tumours or intermediate-risk recurrent tumours with a prior recurrence rate of < 1 recurrence/year and those with a 2006 EORTC recurrence score < 5 benefited from SI. In patients with a 2006 EORTC recurrence score > 5 and/or patients with a prior recurrence rate of > 1 recurrence per year, SI was not effective as a single adjuvant treatment. No randomised comparisons of individual drugs have been conducted [33–36]. Single instillation with mitomycin C (MMC), epirubicin or pirarubicin, have all shown a beneficial effect [33]. Single instillation with gemcitabine was superior to placebo control (saline) in a RCT with approximately 200 patients per arm with remarkably low toxicity rates [37]. These findings are in contrast with a previous study, which, however, used a shorter instillation time [38]. In the Böhle et al. study, continuous saline irrigation was used for 24 hours post-operatively in both arms, which could explain the low recurrence rate in the control arm [38]. Two meta-analyses suggest efficacy of continuous saline irrigation in the prevention of early recurrences [39,40]. Prevention of tumour cell implantation should be initiated within the first few hours after TURB. After that, tumour cells are firmly implanted and are covered by the extracellular matrix [29, 41-43]. In all SI studies, the instillation was administered within 24 hours. Two RCTs found no overall impact of SI with apaziquone; in contrast, a post-hoc analysis did find a reduction of recurrence risk in patients receiving apaziquone within 90 minutes following TURB [44]. To maximise the efficacy of SI, one should devise flexible practices that allow the instillation to be given as soon as possible after TURB, preferably within the first two hours in the recovery room or even in the operating theatre. As severe complications have been reported in patients with drug extravasation [45, 46] safety measures should be maintained.
- Additional adjuvant intravesical chemotherapy instillations
The need for further adjuvant intravesical therapy depends on prognosis. In low-risk patients, a SI reduces the risk of recurrence and is considered to be the standard and complete treatment [33,34]. For other patients, however, a SI remains an incomplete treatment because of the considerable likelihood of recurrence and/or progression. Efficacy data for the following comparisons of application schemes were published:
Single installation only vs. SI and further repeat instillations in one study, further chemotherapy instillations after SI improved RFS in intermediate-risk patients [47]. Repeat chemotherapy instillations vs. no adjuvant treatment A large meta-analysis of 3,703 patients from 11 RCTs showed a highly significant (44%) reduction in the odds of recurrence at one year in favour of chemotherapy over TURB alone [48]. This corresponds to an absolute difference of 13–14% in the number of patients with recurrence. Contrary to these findings, two metaanalyses have demonstrated that BCG therapy may reduce the risk of tumour progression [49, 50]. Moreover, BCG maintenance therapy appears to be significantly better in preventing recurrences than chemotherapy [51–53]. However, BCG causes significantly more side effects than chemotherapy [53]. Single instillation + further repeat instillations vs. later repeat instillations only There is evidence from several studies in intermediate-risk patients that SI might have an impact on recurrence even when further adjuvant instillations are given [54–57]. A RCT including 2,243 NMIBC patients, which compared SI of MMC with an instillation of MMC delayed two weeks after TURB (followed by further repeat instillations in both treatment arms), showed a significant reduction of 9% in the risk of recurrence at 3 years in favour of SI, from 36% to 27%. The effect was significant in the intermediate- and high-risk groups of patients receiving additional adjuvant MMC instillations [54]. Since the author’s definition of the risk groups differed significantly in the initial publication, they adapted their patient stratification in the second analysis and consistently showed improved efficacy of SI followed by repeat MMC instillations [58]. The results of this study should be considered with caution since some patients did not receive adequate therapy. Another RCT found no impact of SI with epirubicin followed by further chemotherapy or BCG instillations in a cohort of predominant HR BC [59]. The optimal schedule of intravesical chemotherapy instillations, the length and frequency of repeat chemotherapy instillations is still controversial; however, it should not exceed one year [57].
- Options for improving efficacy of intravesical chemotherapy
- Adjustment of pH, duration of instillation, and drug concentration
A prospective randomised, multi-institutional RCT showed that intravesical solution reduced the recurrence rate [60]. Another trial reported that duration of a one-hour instillation of MMC was more effective compared to a 30-minute instillation, but no efficacy comparisons are available for one- vs. two-hour durations of instillation [61]. Another RCT using epirubicin has documented that concentration is more important than treatment duration [62]. In view of these data, instructions are provided. It has been suggested that the efficacy of MMC may be improved by optimising application through the adjustment of urine pH, in addition to the use of alternative maintenance schedules. Neither aspect is reflected in the literature quoted above since most published studies do not support this approach.
- Device-assisted intravesical chemotherapy
Microwave-induced hyperthermia effect (RITE) Promising data have been presented on enhancing the efficacy of MMC using microwave-induced hyperthermia in patients with high-risk tumours [63]. In one RCT comparing one year of BCG with one-year MMC and microwave-induced hyperthermia in patients with intermediate- and high-risk BC, increased RFS at 24 months in the MMC group was demonstrated [64].
Hyperthermic intravesical chemotherapy Different technologies which increase the temperature of instilled MMC are available, however, data about their efficacy are still lacking.
Electromotive drug administration
The efficacy of MMC using electromotive drug administration (EMDA) sequentially combined with BCG in patients with high-risk tumours has been demonstrated in one small RCT [65]. The definitive conclusion, however, needs further confirmation. For application of device-assisted instillations in patients recurring after BCG treatment.
Intravesical bacillus Calmette-Guérin (BCG) immunotherapy:
- Efficacy of BCG
- Recurrence rate
Five meta-analyses have confirmed that BCG after TURB is superior to TURB alone or TURB plus chemotherapy for preventing the recurrence of NMIBC [51, 66–69]. Three RCTs of intermediate- and high-risk tumours have compared BCG with epirubicin and interferon (INF) [70], MMC [71], or epirubicin alone [52] and have confirmed the superiority of BCG for prevention of tumour recurrence. The effect is long lasting [52, 71] and was also observed in a separate analysis of patients with intermediate-risk tumours [52]. One meta-analysis [51] has evaluated the individual data from 2,820 patients enrolled in 9 RCTs that have compared MMC vs. BCG. In the trials with BCG maintenance, there was a 32% reduction in the risk of recurrence for BCG compared to MMC, but a 28% increase in the risk of recurrence for patients treated with BCG in the trials without BCG maintenance. A Cochrane systematic review confirmed that BCG is more effective in reducing the recurrence rate over MMC [72].
- Progression rate
Two meta-analyses have demonstrated that BCG therapy delays and potentially lowers the risk of tumour progression [49, 50, 69]. A meta-analysis carried out by the EORTC Genito-Urinary Cancers Group (GUCG) has evaluated data from 4,863 patients enrolled in 24 RCTs. In 20 of the trials, some form of BCG maintenance was used. Based on a median follow-up of 2.5 years, tumours progressed in 9.8% of the patients treated with BCG compared to 13.8% in the control groups (TURB alone, TURB and intravesical chemotherapy, or TURB with the addition of another immunotherapy). This shows a reduction of 27% in the odds of progression with BCG maintenance treatment. The size of the reduction was similar in patients with TaT1 papillary tumours and in those with CIS [50]. A RCT with long-term follow-up has demonstrated significantly fewer distant metastases and better overall- and disease-specific survival in patients treated with BCG compared to epirubicin [52]. In contrast, an IPD meta-analysis and Cochrane review were not able to confirm any statistically significant difference between MMC and BCG for progression, survival, and cause of death [51, 72]. The conflicting results in the outcomes of these studies can be explained by different patient characteristics, duration of follow-up, methodology and statistical power. However, most studies showed a reduction in the risk of progression in high-and intermediate-risk tumours if a BCG maintenance schedule was applied.
- Influence of further factors
Two other meta-analyses have suggested a possible bias in favour of BCG arising from the inclusion of patients previously treated with intravesical chemotherapy [73]. In the IPD meta-analysis, however, BCG maintenance was more effective than MMC in reduction of recurrence rate, both in patients previously treated and not previously treated with chemotherapy [51]. It was demonstrated that BCG was less effective in patients > 70 years of age, but still more effective than epirubicin in a cohort of elderly patients [74]. According to a cohort analysis, the risk of tumour recurrence after BCG was shown to be higher in patients with a previous history of UTUC [75].
- BCG strain
Although smaller studies without maintenance demonstrated some differences between strains [75–77], a network meta-analysis identified ten different BCG strains used for intravesical treatment in the published literature but was not able to confirm superiority of any BCG strain over another [78]. Similarly, a published meta-analysis of prospective RCTs [50], published data from a prospective registry [79] as well as from a post-hoc analysis of a large phase II prospective trial assessing BCG and INF-α in both BCG-naive and BCG-failure patients did not suggest any clear difference in efficacy between the different BCG strains [80]. The quality of data, however, does not allow definitive conclusions.
- BCG toxicity
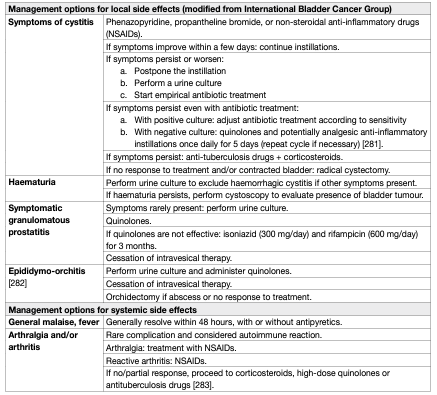
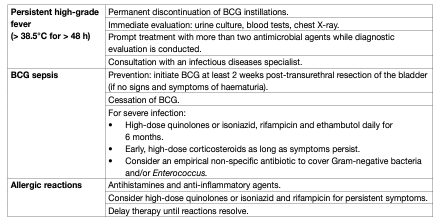
Bacillus Calmette-Guérin intravesical treatment is associated with more side effects compared to intravesical chemotherapy [50,72]. However, serious side effects are encountered in < 5% of patients and can be treated effectively in almost all cases [81]. The incidence of BCG infections after BCG instillations was 1% in a registry-based cohort analysis [82]. It has been shown that a maintenance schedule is not associated with an increased risk of side effects compared to an induction course [81]. Side effects requiring treatment stoppage were seen more often in the first year of therapy [83]. Elderly patients do not seem to experience more side effects leading to treatment discontinuation [84]. No significant difference in toxicity between different BCG strains was demonstrated [79]. Symptoms may be the result of side effects of the BCG treatment or caused by bladder disease (widespread CIS) itself. Consequently, the burden of symptoms is reduced after completion of the treatment in a significant number of patients [85]. Major complications can appear after systemic absorption of the drug. Thus, contraindications of BCG intravesical instillation should be respected. The presence of leukocyturia, nonvisible haematuria or asymptomatic bacteriuria is not a contraindication for BCG application, and antibiotic prophylaxis is not necessary in these cases [86, 87,88]. Bacillus Calmette-Guérin should be used with caution in immunocompromised patients; e.g., immunosuppression, human immunodeficiency virus (HIV) infection poses relative contraindications [89], although some small studies have shown similar efficacy and no increase in complications compared to non-immunocompromised patients. The role of prophylactic anti-tuberculosis medication in these patients remains unclear [90–92]. The management of side effects after BCG should reflect their type and grade according to the recommendations provided by the International Bladder Cancer Group (IBCG) and by a Spanish group [93, 94] (Table 3).
Table 3: Management options for side effects associated with intravesical BCG
- Optimal BCG schedule
Induction BCG instillations are given according to the empirical 6-weekly schedule introduced by Morales et al. [95]. For optimal efficacy, BCG must be given in a maintenance schedule [49–51, 69]. Many different maintenance schedules have been used, ranging from a total of 10 instillations given in 18 to 27 weeks over 3 years [96].
- Optimal number of induction instillations and frequency of instillations during maintenance
The optimal number of induction instillations and frequency of maintenance instillations were evaluated by NIMBUS, a prospective phase III RCT. Safety analysis after 345 randomised patients demonstrated that a reduced number of instillations (3 instillations in induction and 2 instillations at 3, 6 and 12 months) proved inferior to the standard schedule (6 instillation in induction and 3 instillations at 3, 6 and 12 months) regarding the time to first recurrence [97]. In a RCT including 397 patients CUETO showed that in high-risk tumours a maintenance schedule with only one instillation every 3 months for 3 years was not superior to induction therapy only, which suggested that one instillation may be suboptimal to 3 instillations in each maintenance cycle [98].
- Optimal length of maintenance
In their meta-analysis, Böhle et al. concluded that at least one year of maintenance BCG is required to obtain superiority of BCG over MMC for prevention of recurrence or progression [49]. In a RCT of 1,355 patients, the EORTC has shown that when BCG is given at full dose, 3 years’ maintenance (3-weekly instillations 3, 6, 12, 18, 24, 30 and 36 months) reduces the recurrence rate compared to one year in high- but not in intermediate-risk patients. There were no differences in progression or OS. In the 3-year arm, however, 36.1% of patients did not complete the 3-year schedule [99]. The main reason why these patients stopped treatment was treatment inefficacy, not toxicity.
- Optimal dose of BCG
To reduce BCG toxicity, instillation of a reduced dose was proposed. However, it has been suggested that a full dose of BCG is more effective in multifocal tumours [100, 101]. The CUETO study compared one-third dose to full-dose BCG and found no overall difference in efficacy. One-third of the standard dose of BCG might be the minimum effective dose for intermediate-risk tumours. A further reduction to one-sixth dose resulted in a decrease in efficacy with no decrease in toxicity [102]. The EORTC did not find any difference in toxicity between one-third and full-dose BCG, but one-third dose BCG was associated with a higher recurrence rate, especially when it was given only for one year [83, 99]. The routine use of one-third dose BCG is complicated by potential technical difficulties in preparing the reduced dose reliably.
- BCG shortage
A statement by the Panel on BCG shortage can be accessed online: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/?type=appendices-publications.
Combination therapy:
- Intravesical BCG plus chemotherapy versus BCG alone
In one RCT, a combination of MMC and BCG was shown to be more effective in reducing recurrences but more toxic compared to BCG monotherapy. Using similar BCG schedules in both groups, each BCG instillation in the combination group was preceded a day before by one MMC instillation [103]. In a RCT using MMC with EMDA, a combination of BCG and MMC with EMDA showed an improved recurrencefree interval and reduced progression rate compared to BCG monotherapy [65, 104]. Two metaanalyses demonstrated improved disease-free survival (DFS), but no difference in PFS in patients treated with combination treatment comparing to BCG alone [104,105].
- Combination treatment using interferon
In a Cochrane meta-analysis of 4 RCTs, a combination of BCG and IFN-2α did not show a clear difference in recurrence and progression over BCG alone [106]. In one study, weekly MMC followed by monthly BCG alternating with IFN-2α showed a higher probability of recurrence compared to MMC followed by BCG alone [107]. Additionally, a RCT in a similar population of NMIBC comparing BCG monotherapy with a combination of epirubicin and INF for up to two years showed the latter was significantly inferior to BCG monotherapy in preventing recurrence [108].
Specific aspects of treatment of carcinoma in situ:
- Treatment strategy
The detection of concurrent CIS increases the risk of recurrence and progression of TaT1 tumours. Carcinoma in situ cannot be cured by an endoscopic procedure alone. Histological diagnosis of CIS must be followed by further treatment, either intravesical BCG instillations or RC. Tumour-specific survival rates after immediate RC for CIS are excellent, but a large proportion of patients might be over-treated [17].
- Cohort studies on intravesical BCG or chemotherapy
In retrospective evaluations of patients with CIS, a complete response rate of 48% was achieved with intravesical chemotherapy and 72-93% with BCG [17-20, 109]. Up to 50% of complete responders might eventually show recurrence with a risk of invasion and/or extravesical recurrence [20, 43, 96, 109].
- Prospective randomised trials on intravesical BCG or chemotherapy
Unfortunately, there have been few RCTs in patients with CIS only. A meta-analysis of clinical trials comparing intravesical BCG to intravesical chemotherapy in patients with CIS has shown a significantly increased response rate after BCG and a reduction of 59% in the odds of treatment failure with BCG [110]. In an EORTC-GUCG meta-analysis of tumour progression, in a subgroup of 403 patients with CIS, BCG reduced the risk of progression by 35% as compared to intravesical chemotherapy or immunotherapy [50]. The combination of BCG and MMC was not superior to BCG alone [111]. In summary, compared to chemotherapy, BCG treatment of CIS increases the complete response rate, the overall percentage of patients who remain disease free, and reduces the risk of tumour progression.
- Treatment of CIS in the prostatic urethra and upper urinary tract
Patients with CIS are at high risk of extravesical involvement in the UUT and in the prostatic urethra. Solsona et al. found that 63% of 138 patients with CIS developed extravesical involvement initially or during follow-up [112]. Patients with extravesical involvement had worse survival than those with bladder CIS alone [112]. In the prostate, CIS might be present only in the epithelial lining of the prostatic urethra or in the prostatic ducts [113]. These situations should be distinguished from tumour invasion into the prostatic stroma (stage T4a in bladder tumours) and for which immediate radical cystoprostatectomy is mandatory. Patients with CIS in the epithelial lining of the prostatic urethra can be treated by intravesical instillation of BCG. Transurethral resection of the prostate can improve contact of BCG with the prostatic urethra [114, 115]. However, potential spread of CIS has to be considered; no suprapubic trocar-placed catheter should be used. In patients with prostatic duct involvement there are promising results of BCG, but only from small series. The data are insufficient to provide clear treatment recommendations and radical surgery should be considered [115, 116].
Intravesical chemoablation and neoadjuvant treatment:
Older marker lesion studies have shown that chemoablation with a single intravesical chemotherapy instillation can achieve a complete response in a proportion of patients [117]. In addition, hypothesis-generating findings from an older RCT comparing immediate pre-operative device-assisted (EMDA) MMC with post-operative SI with MMC and TURB only, showed improved long-term RFS among patients treated prior to TURB [118], and thus even suggest a long-term effect after neoadjuvant instillations. While this has not been reproduced by other groups, additional neoadjuvant clinical trials were recently published. In recurrent low-risk [119] and recurrent Ta tumours [120], 4 and 6 intravesical MMC instillations achieved complete response in 37% and 57% of the patients, respectively. The former study prematurely stopped recruitment as the anticipated 45% complete response after chemoablation was not achieved. Compared to TURB, less dysuria and incontinence occurred in the intervention arm of the trial. Before routine clinical application, additional high-level evidence with RFS as an outcome measure is required.
Radical cystectomy for non-muscle-invasive bladder cancer:
There are several reasons to consider immediate RC for selected patients with NMIBC:
• The staging accuracy for T1 tumours by TURB is low with 27–51% of patients being upstaged to muscleinvasive tumour at RC [121, 122–126].
• Some patients with NMIBC experience disease progression to muscle-invasive disease (Table 2).
• Patients who experience disease progression to muscle-invasive stage have a worse prognosis than those who present with ‘primary’ muscle-invasive disease [127, 128].
The potential benefit of RC must be weighed against its risks, morbidity, and impact on quality of life (QoL) and discussed with patients, in a shared decision-making process. It is reasonable to propose immediate RC in those patients with NMIBC who are at very high risk of disease progression [129, 130, 131]. Early RC is strongly recommended in patients with BCG-unresponsive tumours and should be considered in BCG relapsing HG tumours as mentioned in Table 4. A delay in RC may lead to decreased disease-specific survival [132]. In patients in whom RC is performed before progression to MIBC, the 5-year DFS rate exceeds 80% [133–135].
Table 4: Treatment options for the various categories of BCG failure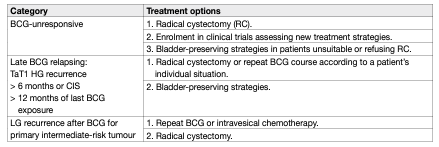
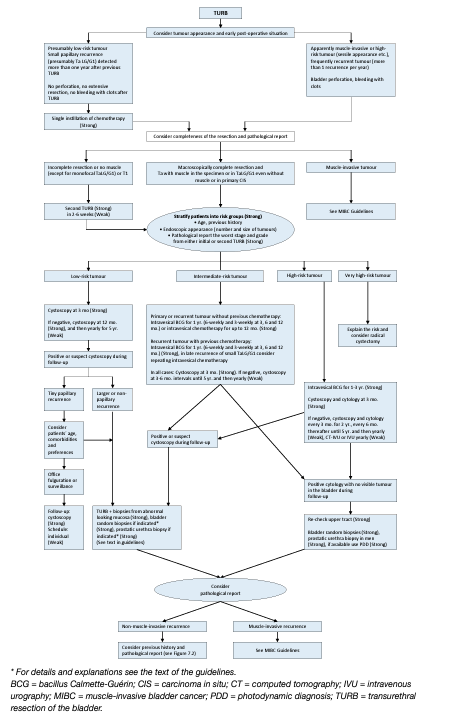
Individual treatment strategy in primary or recurrent tumours after TURB without previous BCG intravesical immunotherapy:
The type of further therapy after TURB should be based on the risk groups shown in Table 1. The stratification and treatment recommendations are based on the risk of disease progression. In particular in intermediate-risk tumours, the 2006 EORTC scoring model may be used as mentioned earlier to determine a patient’s individual risk of disease recurrence as the basis to decide further treatment on. Any decisions should reflect the following principles:
• Patients in the low-risk group have a negligible risk of disease progression. The single post-operative instillation of chemotherapy reduces the risk of recurrence and is considered as sufficient treatment in these patients.
- Patients in the intermediate-risk group have a low risk of disease progression (7.4 and 8.5% after 10 years according to the 2021 EAU NMIBC scoring model). In these patients one-year full-dose BCG treatment (induction plus 3-weekly instillations at 3, 6 and 12 months), or instillations of chemotherapy (the optimal schedule is not known) for a maximum of one year, is recommended. The final choice should reflect the individual patient’s risk of recurrence and progression as well as the efficacy and side effects of each treatment modality.
• Patients in the high-risk group have a high risk of disease progression (14.1 and 14.2% after 10 years according to the 2021 EAU NMIBC scoring model). In these patients full-dose intravesical BCG for one to 3 years (induction plus 3-weekly instillations at 3, 6, 12, 18, 24, 30 and 36 months), is indicated. The additional beneficial effect of the second and third years of maintenance should be weighed against its added costs, side effects and problems associated with BCG shortage. Because of the high risk of progression, immediate RC may also be discussed with the patient. Radical cystectomy is the safest approach from oncological point of view, it is, however, associated with the risk of complications and QoL impairment and represents overtreatment in some patients.
• Patients in the very high-risk group have an extremely high risk of tumour progression (53.1 and 58.6% after 10 years according to the 2021 EAU NMIBC scoring model). Immediate RC should be discussed with these patients. In case RC is not feasible or refused by the patient, full-dose intravesical BCG for one to 3 years should be offered. Figure 1 presents a treatment flow chart based on risk category, which may guide management of an individual patient.
Figure 1: Treatment strategy in primary or recurrent tumour(s) without previous BCG
Treatment of failure of intravesical therapy:
- Recurrence during or after intravesical chemotherapy
Patients with NMIBC recurrence during or after a chemotherapy regimen can benefit from BCG instillations. Prior intravesical chemotherapy has no impact on the effect of BCG instillations [51].
- Treatment failure after intravesical BCG immunotherapy
Several categories of BCG failures, broadly defined as any HG disease occurring during or after BCG therapy, have been proposed (see Table 5). Non-muscle-invasive BC may not respond at all (BCG refractory) or may relapse after initial response (BCG relapsing). Some evidence suggests that patients with BCG relapse have better outcomes than BCG refractory patients [136]. To be able to specify the subgroup of patients where additional BCG is unlikely to provide benefit, the category of BCG-unresponsive tumour was defined. Further BCG instillations in these patients are associated with an increased risk of progression [21, 137]. The category of BCG-unresponsive tumours comprises BCG-refractory and some of BCG-relapsing tumours (see Table 5) [138]. The definition was developed in consultation with the U.S. Food and Drug Administration (FDA), in particular to promote single-arm trials to provide primary evidence of effectiveness in this setting [139]. Non-HG recurrence after BCG is not considered as BCG failure.
Table 5: Categories of high-grade recurrence during or after BCG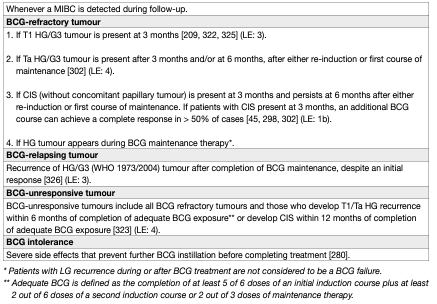
Treatment of BCG-unresponsive tumours, late BCG-relapsing tumours, LG recurrences after BCG treatment and patients with BCG intolerance:
Patients with BCG-unresponsive disease are unlikely to respond to further BCG therapy; RC is therefore the standard and preferred option. Currently, several bladder preservation strategies are being investigated such as cytotoxic intravesical therapies [140–143], device assisted instillations [144–146] intravesical immunotherapy [147, 148], systemic immunotherapy [336] or gene therapy [150–152]. A phase III RCT including predominantly high-risk NMIBC patients failing at least a previous induction course of BCG, MMC combined with microwave-induced hyperthermia provided 35% overall DFS at 2 years as compared to 41% in the control arm (treated with either BCG, MMC or MMC and electromotive drug administration at the discretion of the investigator). In the pre-planned sub-analysis, MMC and microwaveinduced thermotherapy showed lower response rates in CIS recurrences but higher DFS in non-CIS papillary tumours (53% vs. 24%) [146].
Promising data on BCG-unresponsive cohorts of patients with CIS alone or concomitant to papillary tumours were recently reported following new immunotherapies. Systemic pembrolizumab achieved a 40% complete response rate in a prospective phase II study which was maintained in 48% of patients for up to 12 months (n = 101), resulting in FDA approval of the study drug for this patient population [153]. Promising data from a phase III multicentre RCT with intravesical nadofaragene firadenovec were published recently showing a complete response in 53.4% in patients with BCG-unresponsive CIS [154]. A systematic review and meta-analysis including 4 RCTs and 24 single-arm studies (all currently available prospective studies) assessed bladder-sparing treatments following BCG failure [155]. The significant heterogeneity of both trial designs and patient characteristics included in these studies, the different definitions of BCG failures used, and missing information on prior BCG courses may account for the variability in efficacy for the different compounds assessed across different trials. A higher number of previous BCG courses, BCG refractory/unresponsive or CIS predicted lower response rates. The pooled 12-month response rates were 24% for trials with > 2 prior BCG courses and 36% for those with > 1 BCG courses. Initial response rate did not predict durable responses highlighting the need for longer-term follow-up. More recently, a systematic review assessing 42 prospective trials on bladder preserving treatments after BCG showed that patients with papillary-only recurrences appeared more effectively treated (median recurrence free rates of 44% at 1 year, median progression-free rate 89% at a median follow-up of 19 months) than CIS-containing tumours (median complete response rate 17% at 1 year with a median progression-free rate of 95% at a median follow-up of 12 months), highlighting potential biological differences between these two tumour entities which should be analysed separately when reporting results of clinical trials [156]. At the present time, treatments other than RC are considered oncologically inferior in patients with BCGunresponsive disease [21, 137]. Various studies suggest that repeat-BCG therapy is appropriate for non-HG and even for some HG recurrent tumours; namely those relapsing beyond one year after BCG exposure (cases which do not meet the criteria of BCG-unresponsive disease) [157, 158]. Treatment decisions in LG recurrences after BCG (which are not considered as any category of BCG failure) should be individualised according to tumour characteristics. Little is known about the optimal treatment in patients with high-risk tumours who could not complete BCG instillations because of intolerance.
Figure 2: Treatment strategy in recurrence during or after intravesical BCG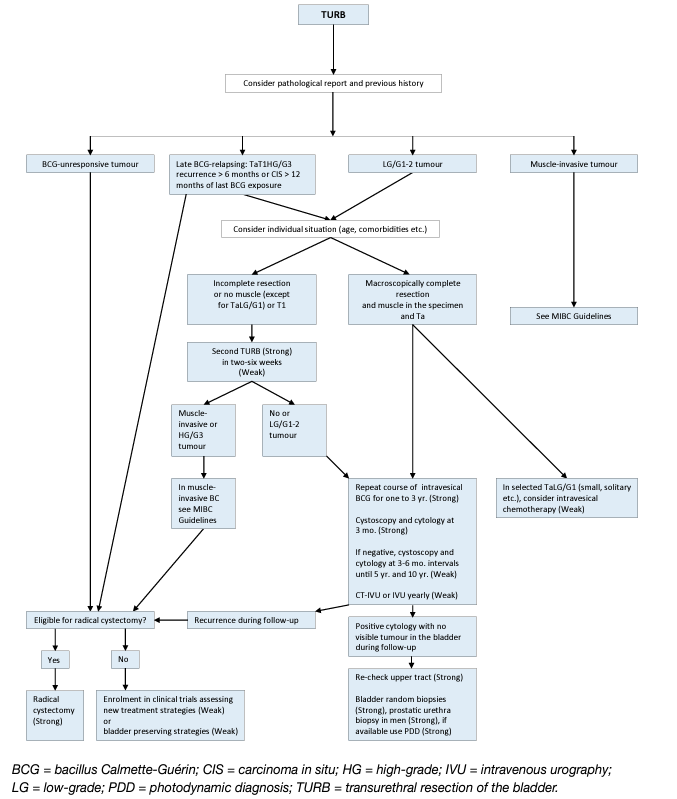
FOLLOW-UP OF PATIENTS WITH NMIBC:
As a result of the risk of recurrence and progression, patients with NMIBC need surveillance following therapy. However, the frequency and duration of cystoscopy and imaging follow-up should reflect the individual patient’s degree of risk. Using the EAU NMIBC prognostic factor risk groups (see Tables 1 and 2) or further prognostic models for specific patient populations.
When planning the follow-up schedule and methods, the following aspects should be considered:
• The prompt detection of muscle-invasive and HG/G3 non-muscle-invasive recurrence is crucial and the percentage of tumours missed should be as low as possible because a delay in diagnosis and therapy can be life-threatening. Therefore, the best surveillance strategy for these patients will continue to include frequent cystoscopy and cytology.
• Tumour recurrence in the low-risk group is nearly always low stage and LG/G1. Small, Ta LG/G1 papillary recurrence does not present an immediate danger to the patient and early detection is not essential for successful therapy [159, 160]. Fulguration of small papillary recurrences on an outpatient basis could be safe [161]. Multiple authors have suggested active surveillance in selected cases [162–164].
• The first cystoscopy after TURB at 3 months is an important prognostic indicator for recurrence and progression [14, 20, 165–167]. Therefore, the first cystoscopy should always be performed 3 months after TURB in all patients with TaT1 tumours and CIS.
• In tumours at low risk, the risk of recurrence after 5 recurrence-free years is low [166]. Therefore, in low-risk tumours, after 5 years of follow-up, discontinuation of cystoscopy or its replacement with lessinvasive methods can be considered [167].
• In tumours originally intermediate-, high risk, or very high risk treated conservatively, recurrences after ten years tumour-free are not unusual [168]. Therefore, life-long follow-up is recommended [167].
• The follow-up strategy must reflect the risk of extravesical recurrence (prostatic urethra in men and UUT in both genders).
• The risk of UUT recurrence increases in patients with multiple- and high-risk tumours [169].
• There may be a role for newer methods of tumour visualisation in follow-up cystoscopy. In a prospective study of blue light flexible cystoscopy (BLFC) for surveillance of NMIBC, BLFC alone showed an abnormality in 8% of which half had biopsy-confirmed BC [170]. On the other hand, a prospective study of narrow-band imaging (NBI) for NMIBC surveillance failed to show any benefit for NBI over white light cystoscopy alone [171].
Non-muscle-invasive BC follow-up strategies include urine cytology and urinary molecular marker tests as adjunct (or companion) tests to improve detection at the time of flexible cystoscopy or as replacement tests to reduce the number of flexible cystoscopies.
• The role of urinary cytology or urinary molecular markers as an adjunct to cystoscopy (companion test) in the follow-up of NMIBC has been investigated [172, 173, 174, 175, 176]. One prospective RCT found that knowledge of positive test results (microsatellite analysis) can improve the quality of follow-up cystoscopy [177].
• In order for urinary markers to reduce or replace cystoscopy altogether, they should be able to detect recurrence across all risk groups. However, currently the limitation of urinary cytology and current urinary markers is their low sensitivity for LG recurrences [178, 179].
• In patients initially diagnosed with Ta LG/G1–2 BC, US of the bladder or a urinary marker may be a mode of surveillance in case cystoscopy is not possible or refused by the patient [180, 181, 182].
• According to current knowledge, no urinary marker can replace cystoscopy during follow-up or lower cystoscopy frequency in a routine fashion.
REFERENCES:
- Sylvester, R.J., et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol, 2006. 49: 466. https://pubmed.ncbi.nlm.nih.gov/16442208/
- Lammers, R.J., et al. Prediction model for recurrence probabilities after intravesical chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer, including external validation. World J Urol, 2016. 34: 173. https://pubmed.ncbi.nlm.nih.gov/26025189/
- Fernandez-Gomez, J., et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol, 2009. 182: 2195. https://pubmed.ncbi.nlm.nih.gov/19758621/
- van Rhijn, B.W., et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol, 2010. 58: 433. https://pubmed.ncbi.nlm.nih.gov/20646825/
- Fernandez-Gomez, J., et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: external validation of the EORTC risk tables. Eur Urol, 2011. 60: 423. https://pubmed.ncbi.nlm.nih.gov/21621906/
- Cambier, S., et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. Eur Urol, 2016. 69: 60. https://pubmed.ncbi.nlm.nih.gov/26210894/
- Sylvester, R.J., et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur Urol, 2021: 480. https://pubmed.ncbi.nlm.nih.gov/33419683/
- Palou, J., et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guerin. Eur Urol, 2012. 62: 118. https://pubmed.ncbi.nlm.nih.gov/22101115/
- Gontero, P., et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: results of a retrospective multicenter study of 2451 patients. Eur Urol, 2015. 67: 74. https://pubmed.ncbi.nlm.nih.gov/25043942/
- Voskuilen, C.S., et al. Urothelial Carcinoma in Bladder Diverticula: A Multicenter Analysis of Characteristics and Clinical Outcomes. Eur Urol Focus, 2020. 6: 1226. https://pubmed.ncbi.nlm.nih.gov/30559065/
- Dalbagni, G., et al. Clinical outcome in a contemporary series of restaged patients with clinical T1 bladder cancer. Eur Urol, 2009. 56: 903. https://pubmed.ncbi.nlm.nih.gov/19632765/
- Bishr, M., et al. Tumour stage on re-staging transurethral resection predicts recurrence and progression-free survival of patients with high-risk non-muscle invasive bladder cancer. Can Urol Assoc J, 2014. 8: E306. https://pubmed.ncbi.nlm.nih.gov/24940455/
- Palou, J., et al. Recurrence, progression and cancer-specific mortality according to stage at re-TUR in T1G3 bladder cancer patients treated with BCG: not as bad as previously thought. World J Urol, 2018. 36: 1621. https://pubmed.ncbi.nlm.nih.gov/29721611/
- Palou, J., et al. Recurrence at three months and high-grade recurrence as prognostic factor of progression in multivariate analysis of T1G2 bladder tumors. Urology, 2009. 73: 1313. https://pubmed.ncbi.nlm.nih.gov/19362341/
- Alkhateeb, S.S., et al. Long-term prognostic value of the combination of EORTC risk group calculator and molecular markers in non-muscle-invasive bladder cancer patients treated with intravesical Bacille Calmette-Guerin. Urol Ann, 2011. 3: 119. https://pubmed.ncbi.nlm.nih.gov/21976923/
- Vartolomei, M.D., et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A systematic review and meta-analysis. Urol Oncol, 2018. 36: 389. https://pubmed.ncbi.nlm.nih.gov/29884342/
- Lamm, D.L. Carcinoma in situ. Urol Clin North Am, 1992. 19: 499. https://pubmed.ncbi.nlm.nih.gov/1636234/
- Losa, A., et al. Low dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: long-term results. J Urol, 2000. 163: 68. https://pubmed.ncbi.nlm.nih.gov/10604316/
- Griffiths, T.R., et al. Treatment of carcinoma in situ with intravesical bacillus Calmette-Guerin without maintenance. J Urol, 2002. 167: 2408. https://pubmed.ncbi.nlm.nih.gov/11992047/
- Takenaka, A., et al. Clinical outcomes of bacillus Calmette-Guerin instillation therapy for carcinoma in situ of urinary bladder. Int J Urol, 2008. 15: 309. https://pubmed.ncbi.nlm.nih.gov/18380817/
- Solsona, E., et al. The 3-month clinical response to intravesical therapy as a predictive factor for progression in patients with high risk superficial bladder cancer. J Urol, 2000. 164: 685. https://pubmed.ncbi.nlm.nih.gov/10953125/
- van Gils-Gielen, R.J., et al. Risk factors in carcinoma in situ of the urinary bladder. Dutch South East Cooperative Urological Group. Urology, 1995. 45: 581. https://pubmed.ncbi.nlm.nih.gov/7716838/
- Lammers, R.J., et al. Smoking status is a risk factor for recurrence after transurethral resection of non-muscle-invasive bladder cancer. Eur Urol, 2011. 60: 713. https://pubmed.ncbi.nlm.nih.gov/21794974/
- Rink, M., et al. Smoking reduces the efficacy of intravesical bacillus Calmette-Guerin immunotherapy in non-muscle-invasive bladder cancer. Eur Urol, 2012. 62: 1204. https://pubmed.ncbi.nlm.nih.gov/22980442/
- Rink, M., et al. Impact of smoking on outcomes of patients with a history of recurrent nonmuscle invasive bladder cancer. J Urol, 2012. 188: 2120. https://pubmed.ncbi.nlm.nih.gov/23083868/
- Crivelli, J.J., et al. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. Eur Urol, 2014. 65: 742. https://pubmed.ncbi.nlm.nih.gov/23810104/
- Muller, J., et al. Trends in the risk of second primary cancer among bladder cancer survivors: a population-based cohort of 10 047 patients. BJU Int, 2016. 118: 53. https://pubmed.ncbi.nlm.nih.gov/26469096/
- Brausi, M., et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol, 2002. 41: 523. https://pubmed.ncbi.nlm.nih.gov/12074794/
- Soloway, M.S., et al. Urothelial susceptibility to tumor cell implantation: influence of cauterization. Cancer, 1980. 46: 1158. https://pubmed.ncbi.nlm.nih.gov/7214299/
- Pan, J.S., et al. Inhibition of implantation of murine bladder tumor by thiotepa in cauterized bladder. J Urol, 1989. 142: 1589. https://pubmed.ncbi.nlm.nih.gov/2511340/
- Brocks, C.P., et al. Inhibition of tumor implantation by intravesical gemcitabine in a murine model of superficial bladder cancer. J Urol, 2005. 174: 1115. https://pubmed.ncbi.nlm.nih.gov/16094076/
- Oosterlinck, W., et al. A prospective European Organization for Research and Treatment of Cancer Genitourinary Group randomized trial comparing transurethral resection followed by a single intravesical instillation of epirubicin or water in single stage Ta, T1 papillary carcinoma of the bladder. J Urol, 1993. 149: 749. https://pubmed.ncbi.nlm.nih.gov/8455236/
- Sylvester, R.J., et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur Urol, 2016. 69: 231. https://pubmed.ncbi.nlm.nih.gov/26091833/
- Sylvester, R.J., et al. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol, 2004. 171: 2186. https://pubmed.ncbi.nlm.nih.gov/15126782/
- Abern, M.R., et al. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. J Natl Compr Canc Netw, 2013. 11: 477. https://pubmed.ncbi.nlm.nih.gov/23584348/
- Perlis, N., et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol, 2013. 64: 421. https://pubmed.ncbi.nlm.nih.gov/23830475/
- Messing, E.M., et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. Jama, 2018. 319: 1880. https://pubmed.ncbi.nlm.nih.gov/29801011/
- Bohle, A., et al. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. Eur Urol, 2009. 56: 495. https://pubmed.ncbi.nlm.nih.gov/19560257/
- Mahran, A., et al. Bladder irrigation after transurethral resection of superficial bladder cancer: a systematic review of the literature. Can J Urol, 2018. 25: 9579. https://pubmed.ncbi.nlm.nih.gov/30553282/
- Zhou, Z., et al. Meta-analysis of efficacy and safety of continuous saline bladder irrigation compared with intravesical chemotherapy after transurethral resection of bladder tumors. World J Urol, 2019. 37: 1075. https://pubmed.ncbi.nlm.nih.gov/30612154/
- Pode, D., et al. The mechanism of human bladder tumor implantation in an in vitro model. J Urol, 1986. 136: 482. https://pubmed.ncbi.nlm.nih.gov/3525861/
- Bohle, A., et al. Inhibition of bladder carcinoma cell adhesion by oligopeptide combinations in vitro and in vivo. J Urol, 2002. 167: 357. https://pubmed.ncbi.nlm.nih.gov/11743356/
- Gofrit, O.N., et al. The natural history of bladder carcinoma in situ after initial response to bacillus Calmette-Guerin immunotherapy. Urol Oncol, 2009. 27: 258. https://pubmed.ncbi.nlm.nih.gov/18440839/
- Karsh, L., et al. Double-Blind, Randomized, Placebo-controlled Studies Evaluating Apaziquone (E09, Qapzola) Intravesical Instillation Post Transurethral Resection of Bladder Tumors for the Treatment of Low-risk Non-Muscle Invasive Bladder Cancer. Bladder Cancer, 2018. 4: 293. https://pubmed.ncbi.nlm.nih.gov/30112440/
- Oddens, J.R., et al. One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? Eur Urol, 2004. 46: 336. https://pubmed.ncbi.nlm.nih.gov/15306104/
- Elmamoun, M.H., et al. Destruction of the bladder by single dose Mitomycin C for low-stage transitional cell carcinoma (TCC)–avoidance, recognition, management and consent. BJU Int, 2014. 113: E34. https://pubmed.ncbi.nlm.nih.gov/24053461/
- Tolley, D.A., et al. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: a further report with 7 years of follow up. J Urol, 1996. 155: 1233. https://pubmed.ncbi.nlm.nih.gov/8632538/
- Huncharek, M., et al. Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer Res, 2001. 21: 765. https://pubmed.ncbi.nlm.nih.gov/11299841/
- Bohle, A., et al. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology, 2004. 63: 682. https://pubmed.ncbi.nlm.nih.gov/15072879/
- Sylvester, R.J., et al. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol, 2002. 168: 1964. https://pubmed.ncbi.nlm.nih.gov/12394686/
- Malmstrom, P.U., et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for nonmuscle-invasive bladder cancer. Eur Urol, 2009. 56: 247. https://pubmed.ncbi.nlm.nih.gov/19409692/
- Sylvester, R.J., et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol, 2010. 57: 766. https://pubmed.ncbi.nlm.nih.gov/20034729/
- Shang, P.F., et al. Intravesical Bacillus Calmette-Guerin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev, 2011: CD006885. https://pubmed.ncbi.nlm.nih.gov/21563157/
- Bosschieter, J., et al. Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. Eur Urol, 2018. 73: 226. https://pubmed.ncbi.nlm.nih.gov/28705539/
- Bouffioux, C., et al. Intravesical adjuvant chemotherapy for superficial transitional cell bladder carcinoma: results of 2 European Organization for Research and Treatment of Cancer randomized trials with mitomycin C and doxorubicin comparing early versus delayed instillations and shortterm versus long-term treatment. European Organization for Research and Treatment of Cancer Genitourinary Group. J Urol, 1995. 153: 934. https://pubmed.ncbi.nlm.nih.gov/7853578/
- Kaasinen, E., et al. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. Eur Urol, 2002. 42: 167. https://pubmed.ncbi.nlm.nih.gov/12160589/
- Sylvester, R.J., et al. The schedule and duration of intravesical chemotherapy in patients with nonmuscle-invasive bladder cancer: a systematic review of the published results of randomized clinical trials. Eur Urol, 2008. 53: 709. https://pubmed.ncbi.nlm.nih.gov/18207317/
- Bosschieter, J., et al. An immediate, single intravesical instillation of mitomycin C is of benefit in patients with non-muscle-invasive bladder cancer irrespective of prognostic risk groups. Urol Oncol, 2018. 36: 400.e7. https://pubmed.ncbi.nlm.nih.gov/30064935/
- Elsawy, A.A., et al. The value of immediate postoperative intravesical epirubicin instillation as an adjunct to standard adjuvant treatment in intermediate and high-risk non-muscle-invasive bladder cancer: A preliminary results of randomized controlled trial. Urol Oncol, 2019. 37: 179 e9. https://pubmed.ncbi.nlm.nih.gov/30448030/
- Au, J.L., et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst, 2001. 93: 597. https://pubmed.ncbi.nlm.nih.gov/11309436/
- Giesbers, A.A., et al. Recurrence of superficial bladder carcinoma after intravesical instillation of mitomycin-C. Comparison of exposure times. Br J Urol, 1989. 63: 176. https://pubmed.ncbi.nlm.nih.gov/2495144/
- Kuroda, M., et al. Effect of prophylactic treatment with intravesical epirubicin on recurrence of superficial bladder cancer–The 6th Trial of the Japanese Urological Cancer Research Group (JUCRG): a randomized trial of intravesical epirubicin at dose of 20mg/40ml, 30mg/40ml, 40mg/40ml. Eur Urol, 2004. 45: 600. https://pubmed.ncbi.nlm.nih.gov/15082202/
- Arends, T.J., et al. Combined chemohyperthermia: 10-year single center experience in 160 patients with nonmuscle invasive bladder cancer. J Urol, 2014. 192: 708. https://pubmed.ncbi.nlm.nih.gov/24704017/
- Arends, T.J., et al. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guerin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. Eur Urol, 2016. 69: 1046. https://pubmed.ncbi.nlm.nih.gov/26803476/
- Di Stasi, S.M., et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol, 2006. 7: 43. https://pubmed.ncbi.nlm.nih.gov/16389183/
- Shelley, M.D., et al. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int, 2001. 88: 209. https://pubmed.ncbi.nlm.nih.gov/11488731/
- Han, R.F., et al. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology, 2006. 67: 1216. https://pubmed.ncbi.nlm.nih.gov/16765182/
- Shelley, M.D., et al. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int, 2004. 93: 485. https://pubmed.ncbi.nlm.nih.gov/15008714/
- Bohle, A., et al. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol, 2003. 169: 90. https://pubmed.ncbi.nlm.nih.gov/12478111/
- Duchek, M., et al. Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferonalpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol, 2010. 57: 25. https://pubmed.ncbi.nlm.nih.gov/19819617/
- Jarvinen, R., et al. Long-term efficacy of maintenance bacillus Calmette-Guerin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up. Eur Urol, 2009. 56: 260. https://pubmed.ncbi.nlm.nih.gov/19395154/
- Schmidt, S., et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev, 2020. 1: Cd011935. https://pubmed.ncbi.nlm.nih.gov/31912907/
- Huncharek, M., et al. The influence of intravesical therapy on progression of superficial transitional cell carcinoma of the bladder: a metaanalytic comparison of chemotherapy versus bacilli CalmetteGuerin immunotherapy. Am J Clin Oncol, 2004. 27: 522. https://pubmed.ncbi.nlm.nih.gov/15596924/
- Oddens, J.R., et al. The effect of age on the efficacy of maintenance bacillus calmette-guerin relative to maintenance epirubicin in patients with stage ta t1 urothelial bladder cancer: results from EORTC genito-urinary group study 30911. Eur Urol, 2014. 66: 694. https://pubmed.ncbi.nlm.nih.gov/24948466/
- Miyake, M., et al. Outcomes of subsequent non-muscle-invasive bladder cancer treated with intravesical Bacillus Calmette-Guerin after radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int, 2018. 121: 764. https://pubmed.ncbi.nlm.nih.gov/29281857/
- Rentsch, C.A., et al. Bacillus calmette-guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol, 2014. 66: 677. https://pubmed.ncbi.nlm.nih.gov/24674149/
- Sengiku, A., et al. A prospective comparative study of intravesical bacillus Calmette-Guerin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol, 2013. 190: 50. https://pubmed.ncbi.nlm.nih.gov/23376145/
- Boehm, B.E., et al. Efficacy of bacillus Calmette-Guerin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. J Urol, 2017. 198: 503. https://pubmed.ncbi.nlm.nih.gov/28286068/
- Unda-Urzaiz, M., et al. Safety and efficacy of various strains of bacille Calmette-Guerin in the treatment of bladder tumours in standard clinical practice. Actas Urol Esp, 2018. 42: 238. https://pubmed.ncbi.nlm.nih.gov/29295749/
- Steinberg, R.L., et al. Bacillus Calmette-Guerin strain may not effect recurrence-free survival when used intravesically with interferon-alpha2b for non-muscle-invasive bladder cancer. Urol Oncol, 2017. 35: 201. https://pubmed.ncbi.nlm.nih.gov/28041998/
- van der Meijden, A.P., et al. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol, 2003. 44: 429. https://pubmed.ncbi.nlm.nih.gov/14499676/
- Larsen, E.S., et al. The epidemiology of bacille Calmette-Guerin infections after bladder instillation from 2002 through 2017: a nationwide retrospective cohort study. BJU Int, 2019. https://pubmed.ncbi.nlm.nih.gov/31054198/
- Brausi, M., et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol, 2014. 65: 69. https://pubmed.ncbi.nlm.nih.gov/23910233/
- Oddens, J.R., et al. Increasing age is not associated with toxicity leading to discontinuation of treatment in patients with urothelial non-muscle-invasive bladder cancer randomised to receive 3 years of maintenance bacille Calmette-Guerin: results from European Organisation for Research and Treatment of Cancer Genito-Urinary Group study 30911. BJU Int, 2016. 118: 423. https://pubmed.ncbi.nlm.nih.gov/26945890/
- Danielsson, G., et al. Bladder health in patients treated with BCG instillations for T1G2-G3 bladder cancer – a follow-up five years after the start of treatment. Scand J Urol, 2018. 52: 377. https://pubmed.ncbi.nlm.nih.gov/
- 30616479/ Herr, H.W. Intravesical bacillus Calmette-Guerin outcomes in patients with bladder cancer and asymptomatic bacteriuria. J Urol, 2012. 187: 435. https://pubmed.ncbi.nlm.nih.gov/22177154/
- Herr, H.W. Outpatient urological procedures in antibiotic-naive patients with bladder cancer with asymptomatic bacteriuria. BJU Int, 2012. 110: E658. https://pubmed.ncbi.nlm.nih.gov/22883017/
- Critelli, R., et al. Detection of multiple mutations in urinary exfoliated cells from male bladder cancer patients at diagnosis and during follow-up. Oncotarget, 2016. 7: 67435. https://pubmed.ncbi.nlm.nih.gov/27611947/
- Lamm, D.L., et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol, 1992. 147: 596. https://pubmed.ncbi.nlm.nih.gov/1538436/
- Palou, J., et al. Intravesical bacillus Calmette-Guerin for the treatment of superficial bladder cancer in renal transplant patients. Transplantation, 2003. 76: 1514. https://pubmed.ncbi.nlm.nih.gov/14657696/
- Yossepowitch, O., et al. Safety and efficacy of intravesical bacillus Calmette-Guerin instillations in steroid treated and immunocompromised patients. J Urol, 2006. 176: 482. https://pubmed.ncbi.nlm.nih.gov/16813873/
- Roumeguere, T., et al. Bacillus Calmette-Guerin therapy in non-muscle-invasive bladder carcinoma after renal transplantation for end-stage aristolochic acid nephropathy. Transpl Int, 2015. 28: 199. https://pubmed.ncbi.nlm.nih.gov/25377421/
- Rodriguez, F., et al. [Practical guideline for the management of adverse events associated with BCG installations]. Arch Esp Urol, 2008. 61: 591. https://pubmed.ncbi.nlm.nih.gov/18709813/
- Witjes J.A., et al. Clinical practice recommendations for the prevention and management of intravesical therapy-associated adverse events. Eur Urol Suppl, 2008. 7: 667. https://www.sciencedirect.com/science/article/abs/pii/S1569905608001103
- Morales, A., et al. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol, 1976. 116: 180. https://pubmed.ncbi.nlm.nih.gov/820877/
- Lamm, D.L., et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol, 2000. 163: 1124. https://pubmed.ncbi.nlm.nih.gov/10737480/
- Grimm, M.O., et al. Treatment of High-grade Non-muscle-invasive Bladder Carcinoma by Standard Number and Dose of BCG Instillations Versus Reduced Number and Standard Dose of BCG Instillations: Results of the European Association of Urology Research Foundation Randomised Phase III Clinical Trial “NIMBUS”. Eur Urol, 2020. 78: 690. https://pubmed.ncbi.nlm.nih.gov/32446864/
- Martinez-Pineiro, L., et al. Maintenance Therapy with 3-monthly Bacillus Calmette-Guerin for 3 Years is Not Superior to Standard Induction Therapy in High-risk Non-muscle-invasive Urothelial Bladder Carcinoma: Final Results of Randomised CUETO Study 98013. Eur Urol, 2015. 68: 256. https://pubmed.ncbi.nlm.nih.gov/25794457/
- Oddens, J., et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol, 2013. 63: 462. https://pubmed.ncbi.nlm.nih.gov/23141049/
- Martinez-Pineiro, J.A., et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guerin with a reduced dose of 27 mg in superficial bladder cancer. BJU Int, 2002. 89: 671. https://pubmed.ncbi.nlm.nih.gov/11966623/
- Martinez-Pineiro, J.A., et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol, 2005. 174: 1242. https://pubmed.ncbi.nlm.nih.gov/16145378/
- Ojea, A., et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. Eur Urol, 2007. 52: 1398. https://pubmed.ncbi.nlm.nih.gov/17485161/
- Solsona, E., et al. Sequential combination of mitomycin C plus bacillus Calmette-Guerin (BCG) is more effective but more toxic than BCG alone in patients with non-muscle-invasive bladder cancer in intermediate- and high-risk patients: final outcome of CUETO 93009, a randomized prospective trial. Eur Urol, 2015. 67: 508. https://pubmed.ncbi.nlm.nih.gov/25301758/
- Cui, J., et al. Combination of Intravesical Chemotherapy and Bacillus Calmette-Guerin Versus Bacillus Calmette-Guerin Monotherapy in Intermediate- and High-risk Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-analysis. Medicine (Baltimore), 2016. 95: e2572. https://pubmed.ncbi.nlm.nih.gov/26817914/
- Huang, D., et al. Combination of Intravesical Bacille Calmette-Guerin and Chemotherapy vs. Bacille Calmette-Guerin Alone in Non-muscle Invasive Bladder Cancer: A Meta-Analysis. Front Oncol, 2019. 9: 121. https://pubmed.ncbi.nlm.nih.gov/30881921/
- Shepherd, A.R., et al. Intravesical Bacillus Calmette-Guerin with interferon-alpha versus intravesical Bacillus Calmette-Guerin for treating non-muscle-invasive bladder cancer. Cochrane Database Syst Rev, 2017. 3: CD012112. https://pubmed.ncbi.nlm.nih.gov/28268259/
- Jarvinen, R., et al. Long-term outcome of patients with frequently recurrent non-muscle-invasive bladder carcinoma treated with one perioperative plus four weekly instillations of mitomycin C followed by monthly bacillus Calmette-Guerin (BCG) or alternating BCG and interferon-alpha2b instillations: prospective randomised FinnBladder-4 study. Eur Urol, 2015. 68: 611. https://pubmed.ncbi.nlm.nih.gov/25748117/
- Marttila, T., et al. Intravesical Bacillus Calmette-Guerin Versus Combination of Epirubicin and Interferon-alpha2a in Reducing Recurrence of Non-Muscle-invasive Bladder Carcinoma: FinnBladder-6 Study. Eur Urol, 2016. 70: 341. https://pubmed.ncbi.nlm.nih.gov/27085624/
- Jakse, G., et al. Intravesical BCG in patients with carcinoma in situ of the urinary bladder: long-term results of EORTC GU Group phase II protocol 30861. Eur Urol, 2001. 40: 144. https://pubmed.ncbi.nlm.nih.gov/11528191/
- Sylvester, R.J., et al. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol, 2005. 174: 86. https://pubmed.ncbi.nlm.nih.gov/15947584/ K
- aasinen, E., et al. Seventeen-year follow-up of the prospective randomized Nordic CIS study: BCG monotherapy versus alternating therapy with mitomycin C and BCG in patients with carcinoma in situ of the urinary bladder. Scand J Urol, 2016. 50: 360. https://pubmed.ncbi.nlm.nih.gov/27603424/
- Solsona, E., et al. Extravesical involvement in patients with bladder carcinoma in situ: biological and therapy implications. J Urol, 1996. 155: 895. https://pubmed.ncbi.nlm.nih.gov/8583601/
- Sylvester, R.J., et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology, 2005. 66: 90. https://pubmed.ncbi.nlm.nih.gov/16399418/
- Aaronson, D.S., et al. Meta-analysis: does lidocaine gel before flexible cystoscopy provide pain relief? BJU Int, 2009. 104: 506. https://pubmed.ncbi.nlm.nih.gov/19239453/
- Palou, J., et al. Urothelial carcinoma of the prostate. Urology, 2007. 69: 50. https://pubmed.ncbi.nlm.nih.gov/17280908/
- Palou Redorta, J., et al. Intravesical instillations with bacillus calmette-guerin for the treatment of carcinoma in situ involving prostatic ducts. Eur Urol, 2006. 49: 834. https://pubmed.ncbi.nlm.nih.gov/16426729/
- Popert, R.J., et al. Superficial bladder cancer: the response of a marker tumour to a single intravesical instillation of epirubicin. Br J Urol, 1994. 74: 195. https://pubmed.ncbi.nlm.nih.gov/7921938/
- Di Stasi, S.M., et al. Electromotive instillation of mitomycin immediately before transurethral resection for patients with primary urothelial non-muscle invasive bladder cancer: a randomised controlled trial. Lancet Oncol, 2011. 12: 871. https://pubmed.ncbi.nlm.nih.gov/21831711/
- Mostafid, A.H., et al. CALIBER: a phase II randomized feasibility trial of chemoablation with mitomycin-C vs surgical management in low-risk non-muscle-invasive bladder cancer. BJU Int, 2020. 125: 817. https://pubmed.ncbi.nlm.nih.gov/32124514/
- Lindgren, M.S., et al. The DaBlaCa-13 Study: Short-term, Intensive Chemoresection Versus Standard Adjuvant Intravesical Instillations in Non-muscle-invasive Bladder Cancer-A Randomised Controlled Trial. Eur Urol, 2020. 78: 856. https://pubmed.ncbi.nlm.nih.gov/32736928/
- Huguet, J., et al. Cystectomy in patients with high risk superficial bladder tumors who fail intravesical BCG therapy: pre-cystectomy prostate involvement as a prognostic factor. Eur Urol, 2005. 48: 53. https://pubmed.ncbi.nlm.nih.gov/15967252/
- Fritsche, H.M., et al. Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. Eur Urol, 2010. 57: 300. https://pubmed.ncbi.nlm.nih.gov/19766384/
- Turker, P., et al. Upstaging of urothelial cancer at the time of radical cystectomy: factors associated with upstaging and its effect on outcome. BJU Int, 2012. 110: 804. https://pubmed.ncbi.nlm.nih.gov/22321341/
- May, M., et al. Pathological upstaging detected in radical cystectomy procedures is associated with a significantly worse tumour-specific survival rate for patients with clinical T1 urothelial carcinoma of the urinary bladder. Scand J Urol Nephrol, 2011. 45: 251. https://pubmed.ncbi.nlm.nih.gov/21388337/
- Svatek, R.S., et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int, 2011. 107: 898. https://pubmed.ncbi.nlm.nih.gov/21244604/
- Shariat, S.F., et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol, 2007. 51: 137. https://pubmed.ncbi.nlm.nih.gov/16793197/
- Moschini, M., et al. Comparing long-term outcomes of primary and progressive carcinoma invading bladder muscle after radical cystectomy. BJU Int, 2016. 117: 604. https://pubmed.ncbi.nlm.nih.gov/25851271/
- Schrier, B.P., et al. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol, 2004. 45: 292. https://pubmed.ncbi.nlm.nih.gov/15036673/
- Willis, D.L., et al. Clinical outcomes of cT1 micropapillary bladder cancer. J Urol, 2015. 193: 1129. https://pubmed.ncbi.nlm.nih.gov/25254936/
- Palou, J., et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guerin. Eur Urol, 2012. 62: 118. https://pubmed.ncbi.nlm.nih.gov/22101115/
- Kamat, A.M., et al. The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J Urol, 2006. 175: 881. https://pubmed.ncbi.nlm.nih.gov/16469571/
- Raj, G.V., et al. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J Urol, 2007. 177: 1283. https://pubmed.ncbi.nlm.nih.gov/17382713/
- Stein, J.P., et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol, 2001. 19: 666. https://pubmed.ncbi.nlm.nih.gov/11157016/
- Hautmann, R.E., et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol, 2012. 61: 1039. https://pubmed.ncbi.nlm.nih.gov/22381169/
- Shariat, S.F., et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol, 2006. 176: 2414. https://pubmed.ncbi.nlm.nih.gov/17085118/
- Herr, H.W., et al. BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol Oncol, 2015. 33: 108.e1. https://pubmed.ncbi.nlm.nih.gov/25813144/
- Lerner, S.P., et al. Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol Oncol, 2009. 27: 155. https://pubmed.ncbi.nlm.nih.gov/18367117/
- Kamat, A.M., et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol, 2016. 34: 1935. https://pubmed.ncbi.nlm.nih.gov/26811532/
- U.S. Food and Drug Administration (FDA). BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment. Guidance for Industry. Center for Drug Evaluation and Research (CDER). 2018. https://www.fda.gov/media/101468/download
- Cockerill, P.A., et al. Intravesical gemcitabine in combination with mitomycin C as salvage treatment in recurrent non-muscle-invasive bladder cancer. BJU Int, 2016. 117: 456. https://pubmed.ncbi.nlm.nih.gov/25682834/
- Barlow, L., et al. A single-institution experience with induction and maintenance intravesical docetaxel in the management of non-muscle-invasive bladder cancer refractory to bacille CalmetteGuerin therapy. BJU Int, 2009. 104: 1098. https://pubmed.ncbi.nlm.nih.gov/19389012/
- Steinberg, G., et al. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol, 2000. 163: 761. https://pubmed.ncbi.nlm.nih.gov/10687972/
- Jones, G., et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev, 2012. 1: CD009294. https://pubmed.ncbi.nlm.nih.gov/22259002/
- Nativ, O., et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. J Urol, 2009. 182: 1313. https://pubmed.ncbi.nlm.nih.gov/19683278/
- Racioppi, M., et al. ElectroMotive drug administration (EMDA) of Mitomycin C as first-line salvage therapy in high risk “BCG failure” non muscle invasive bladder cancer: 3 years follow-up outcomes. BMC Cancer, 2018. 18: 1224. https://pubmed.ncbi.nlm.nih.gov/30522445/
- Tan, W.S., et al. Radiofrequency-induced Thermo-chemotherapy Effect Versus a Second Course of Bacillus Calmette-Guerin or Institutional Standard in Patients with Recurrence of Non-muscleinvasive Bladder Cancer Following Induction or Maintenance Bacillus Calmette-Guerin Therapy (HYMN): A Phase III, Open-label, Randomised Controlled Trial. Eur Urol, 2019. 75: 63. https://pubmed.ncbi.nlm.nih.gov/30274699/
- Morales, A., et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette-Guerin. J Urol, 2015. 193: 1135. https://pubmed.ncbi.nlm.nih.gov/25286009/
- Joudi, F.N., et al. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol, 2006. 24: 344. https://pubmed.ncbi.nlm.nih.gov/16818189/
- Wright, K.M. FDA Approves Pembrolizumab for BCG-Unresponsive NMIBC. Oncology (Williston Park), 2020. 34: 44. https://pubmed.ncbi.nlm.nih.gov/32645193/
- Shore, N.D., et al. Intravesical rAd-IFNalpha/Syn3 for Patients With High-Grade, Bacillus CalmetteGuerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol, 2017. 35: 3410. https://pubmed.ncbi.nlm.nih.gov/28834453/
- Packiam, V.T., et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol, 2018. 36: 440. https://pubmed.ncbi.nlm.nih.gov/28755959/
- Hassler, M.R., et al. Salvage therapeutic strategies for bacillus Calmette-Guerin failure. Curr Opin Urol, 2019. 29: 239. https://pubmed.ncbi.nlm.nih.gov/30762670/
- Balar, A.V., et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol, 2021. 22: 919. https://pubmed.ncbi.nlm.nih.gov/34051177/
- Boorjian, S.A., et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol, 2021. 22: 107. https://pubmed.ncbi.nlm.nih.gov/33253641/
- Kamat, A.M., et al. Evidence-based Assessment of Current and Emerging Bladder-sparing Therapies for Non-muscle-invasive Bladder Cancer After Bacillus Calmette-Guerin Therapy: A Systematic Review and Meta-analysis. Eur Urol Oncol, 2020. 3: 318. https://pubmed.ncbi.nlm.nih.gov/32201133/
- Li, R., et al. Systematic Review of the Therapeutic Efficacy of Bladder-preserving Treatments for Non-muscle-invasive Bladder Cancer Following Intravesical Bacillus Calmette-Guérin. Eur Urol, 2020. 78: 387. https://pubmed.ncbi.nlm.nih.gov/32143924/
- Gallagher, B.L., et al. Impact of previous bacille Calmette-Guerin failure pattern on subsequent response to bacille Calmette-Guerin plus interferon intravesical therapy. Urology, 2008. 71: 297. https://pubmed.ncbi.nlm.nih.gov/18308107/
- Rosevear, H.M., et al. Factors affecting response to bacillus Calmette-Guerin plus interferon for urothelial carcinoma in situ. J Urol, 2011. 186: 817. https://pubmed.ncbi.nlm.nih.gov/21788050/
- Holmang, S., et al. Stage progression in Ta papillary urothelial tumors: relationship to grade, immunohistochemical expression of tumor markers, mitotic frequency and DNA ploidy. J Urol, 2001. 165: 1124. https://pubmed.ncbi.nlm.nih.gov/11257652/
- Gofrit, O.N., et al. Watchful waiting policy in recurrent Ta G1 bladder tumors. Eur Urol, 2006. 49: 303. https://pubmed.ncbi.nlm.nih.gov/16413659/
- Herr, H.W., et al. Management of low grade papillary bladder tumors. J Urol, 2007. 178: 1201. https://pubmed.ncbi.nlm.nih.gov/17698090/
- Pruthi, R.S., et al. Conservative management of low risk superficial bladder tumors. J Urol, 2008. 179: 87. https://pubmed.ncbi.nlm.nih.gov/17997444/
- Hernandez, V., et al. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol, 2016. 34: 165 e19. https://pubmed.ncbi.nlm.nih.gov/26687318/
- Hurle, R., et al. Active Surveillance for Low Risk Nonmuscle Invasive Bladder Cancer: A Confirmatory and Resource Consumption Study from the BIAS Project. J Urol, 2018. 199: 401. https://pubmed.ncbi.nlm.nih.gov/28847481/
- Holmang, S., et al. Stage Ta-T1 bladder cancer: the relationship between findings at first followup cystoscopy and subsequent recurrence and progression. J Urol, 2002. 167: 1634. https://pubmed.ncbi.nlm.nih.gov/11912378/
- Mariappan, P., et al. A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. J Urol, 2005. 173: 1108. https://pubmed.ncbi.nlm.nih.gov/15758711/
- Soukup, V., et al. Follow-up after surgical treatment of bladder cancer: a critical analysis of the literature. Eur Urol, 2012. 62: 290. https://pubmed.ncbi.nlm.nih.gov/22609313/
- Holmang, S., et al. Should follow-up cystoscopy in bacillus Calmette-Guerin-treated patients continue after five tumour-free years? Eur Urol, 2012. 61: 503. https://pubmed.ncbi.nlm.nih.gov/22119022/
- Millan-Rodriguez, F., et al. Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J Urol, 2000. 164: 1183. https://pubmed.ncbi.nlm.nih.gov/10992362/
- Lotan, Y., et al. Prospective evaluation of blue-light flexible cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer. BJU Int, 2021. 127: 108. https://pubmed.ncbi.nlm.nih.gov/32648957/
- Tschirdewahn, S., et al. Narrow-band imaging assisted cystoscopy in the follow-up of patients with transitional cell carcinoma of the bladder: a randomized study in comparison with white light cystoscopy. World J Urol, 2020. 38: 1509. https://pubmed.ncbi.nlm.nih.gov/31471739/
- Kavalieris, L., et al. Performance Characteristics of a Multigene Urine Biomarker Test for Monitoring for Recurrent Urothelial Carcinoma in a Multicenter Study. J Urol, 2017. 197: 1419. https://pubmed.ncbi.nlm.nih.gov/27986532/
- Beukers, W., et al. FGFR3, TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. J Urol, 2017. 197: 1410. https://pubmed.ncbi.nlm.nih.gov/28049011/
- Valenberg, F., et al. Prospective Validation of an mRNA-based Urine Test for Surveillance of Patients with Bladder Cancer. Eur Urol, 2019. 75: 853. https://pubmed.ncbi.nlm.nih.gov/30553612/
- D’Andrea, D., et al. Diagnostic accuracy, clinical utility and influence on decision-making of a methylation urine biomarker test in the surveillance of non-muscle-invasive bladder cancer. BJU Int, 2019. 123: 959. https://pubmed.ncbi.nlm.nih.gov/30653818/
- Babjuk, M., et al. Urinary cytology and quantitative BTA and UBC tests in surveillance of patients with pTapT1 bladder urothelial carcinoma. Urology, 2008. 71: 718. https://pubmed.ncbi.nlm.nih.gov/18387400/
- van der Aa, M.N., et al. Cystoscopy revisited as the gold standard for detecting bladder cancer recurrence: diagnostic review bias in the randomized, prospective CEFUB trial. J Urol, 2010. 183: 76. https://pubmed.ncbi.nlm.nih.gov/19913254/
- van Rhijn, B.W., et al. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol, 2005. 47: 736. https://pubmed.ncbi.nlm.nih.gov/15925067/
- Beukers, W., et al. FGFR3, TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. J Urol, 2017. 197: 1410. https://pubmed.ncbi.nlm.nih.gov/28049011/
- Roobol, M.J., et al. Feasibility study of screening for bladder cancer with urinary molecular markers (the BLU-P project). Urol Oncol, 2010. 28: 686. https://pubmed.ncbi.nlm.nih.gov/21062653/
- Babjuk, M., et al. Urinary cytology and quantitative BTA and UBC tests in surveillance of patients with pTapT1 bladder urothelial carcinoma. Urology, 2008. 71: 718. https://pubmed.ncbi.nlm.nih.gov/18387400/
- Niwa, N., et al. Comparison of outcomes between ultrasonography and cystoscopy in the surveillance of patients with initially diagnosed TaG1-2 bladder cancers: A matched-pair analysis. Urol Oncol, 2015. 33: 386 e15. https://pubmed.ncbi.nlm.nih.gov/26027764/
Author Correspondence:
Prof. Dr. Semir A. Salim. Al Samarrai
Medical Director of Professor Al Samarrai Medical Center.
Dubai Healthcare City, Al-Razi Building 64, Block D, 2nd Floor, Suite 2018
E-mail: semiralsamarrai@hotmail.com
Tel: +97144233669

