EPIDEMIOLOGY AND PREVALENCE OF
SEXUAL DYSFUNCTION AND DISORDERS OF
MALE REPRODUCTIVE HEALTH
Prof. Dr. Semir. A. Salim. Al Samarrai
Erectile dysfunction:
Epidemiological data have shown a high prevalence and incidence of ED worldwide [1]. Among others, the Massachusetts Male Aging Study (MMAS) [2] reported an overall prevalence of 52% ED in non-institutionalized men aged 40-70 years in the Boston area; specific prevalence for minimal, moderate, and complete ED was 17.2%, 25.2%, and 9.6%, respectively. In the Cologne study of men aged 30-80 years, the prevalence of ED was 19.2%, with a steep age-related increase from 2.3% to 53.4% [3]. The incidence rate of ED (new cases per 1,000 men annually) was 26 in the long-term data from the MMAS study [4] and 19.2 (mean follow-up of 4.2 years) in a Dutch study [5]. In a cross-sectional real-life study among men seeking first medical help for new-onset ED, one in four patients was younger than 40 years, with almost 50% of the young men complaining of severe ED [6]. Differences among these studies can be explained by differences in methodology, ages, and socio-economic and cultural status of the populations studied.
Premature ejaculation:
The method of recruitment for study participation, method of data collection and operational criteria can all greatly affect reported prevalence rates of premature ejaculation (PE). The major problem in assessing the prevalence of PE was the lack of a universally recognised definition at the time the surveys were conducted [7]. Vague definitions without specific operational criteria, different manners of sampling, and non-standardized data acquisition have led to heterogeneity in estimated prevalence [7–11]. The highest prevalence rate of 31% (men aged 18-59 years) was found by the National Health and Social Life Survey (NHSLS), which determines adult sexual behaviour in the USA [12]. Prevalence rates were 30% (18-29 years), 32% (30-39 years), 28% (40-49 years) and 55% (50-59 years). It is, however, unlikely that the PE prevalence is as high as 20-30% based on the relatively low number of men who seek medical help for PE. These high prevalence rates may be a result of the dichotomous scale (yes/no) in a single question asking if ejaculation occurred too early, as the prevalence rates in European studies have been significantly lower [13]. Two separate observational, cross-sectional surveys from different continents found that overall prevalence of PE was 19.8 and 25.8%, respectively [14,15]. Further stratifying these complaints into the classifications defined by Waldinger et al. [16], rates of lifelong PE were 2.3 and 3.18%, acquired PE 3.9 and 4.48%, variable PE 8.5 and 11.38% and subjective PE 5.1 and 6.4% [14,15]. Both studies showed that men with acquired PE were more likely to seek treatment compared to men with lifelong PE. Treatment-seeking behaviour may have contributed to errors in the previously reported rates of PE, as it is possible that men with lifelong PE came to terms with their problem and did not seek treatment. The additional psychological burden of a new change in ejaculatory latency in acquired PE may have prompted more frequent treatment seeking [17]. Thus, it is likely that there is disparity between the incidence of the various PE sub-types in the general community and in men actively seeking treatment for PE [18,19]. This disparity could be a further barrier to understanding the true incidence of each sub-type of PE. An approximately 5% prevalence of acquired PE and lifelong PE in the general population is consistent with epidemiological data indicating that around 5% of the population have an ejaculation latency of < 2 minutes [20].
Other ejaculatory disorders:
- Delayed ejaculation
Due to its rarity and uncertain definitions, the epidemiology of delayed ejaculation (DE) is not clear [21]. However, several well-designed epidemiological studies have revealed that its prevalence is around 3% among sexually active men [12,22]. According to data from the NHSLS, 7.78% of a national probability sample of 1,246 men aged 18-59 years reported inability achieving climax or ejaculation [12]. In a similar stratified national probability sample survey completed over 6 months among 11,161 men and women aged 16-44 years in Britain, 0.7% of men reported inability to reach orgasm [23]. In an international survey of sexual problems among 13,618 men aged 40–80 years from 29 countries, 1.1-2.8% of men reported that they frequently experience inability to reach orgasm [24]. Another study conducted in the United States (USA), in a national probability sample of 1,455 men aged 57-85 years, 20% of men reported inability to climax and 73% reported that they were bothered by this problem [25]. Considering the findings of these epidemiological studies and their clinical experiences, some urologists and sex therapists have postulated that the prevalence of DE may be higher among older men [26-28]. Similar to the general population, the prevalence of men with DE is low among patients who seek treatment for their sexual problems. An Indian study that evaluated the data on 1,000 consecutive patients with sexual disorders who attended a psychosexual clinic demonstrated that the prevalence of DE was 0.6% and it was more frequent in elderly people with diabetes [29]. Nazareth et al. [30] evaluated the prevalence of International Classification of Diseases 10th edition (ICD-10) diagnosed sexual dysfunctions among 447 men attending 13 general practices in London, UK and found that 2.5% of the men reported inhibited orgasm during intercourse. Similar to PE, there are distinctions among lifelong, acquired and situational DE [31]. Although the evidence is limited, the prevalence of lifelong and acquired DE is estimated at 1 and 4%, respectively [32].
- Anejaculation and Anorgasmia
Establishing the exact prevalence of anejaculation and anorgasmia is difficult since many men cannot distinguish between ejaculation and orgasm. The rarity of these clinical conditions further hampers the attempts to conduct epidemiological studies. In a report from the USA, 8% of men reported unsuccessfully achieving orgasm during the past year [12]. According to Kinsey et al. [33], 0.14% of the general population have anejaculation. The most common causes of anejaculation were spinal cord injury, diabetes mellitus and multiple sclerosis. Especially in most cases of spinal cord injury, medical assistance is the only way to ejaculate. While masturbation leads to the lowest rates of ejaculation, higher response rates can be obtained with penile vibratory stimulation or acetylcholine esterase inhibitors followed by masturbation in patients with spinal cord injury [34].
- Retrograde ejaculation
Similar to anejaculation, it is difficult to estimate the true incidence of retrograde ejaculation (RE). Although RE is generally reported in 0.3-2% of patients attending fertility clinics [35], diabetes may increase these rates by leading to autonomic neuropathy. Autonomic neuropathy results in ED and ejaculatory dysfunctions ranging from DE to RE and anejaculation, depending on the degree of sympathetic autonomic neuropathy involved [36]. In 54 diabetic patients with sexual dysfunction, RE was observed with a 6% incidence [37]. In a controlled trial, RE was observed in 34.6% of diabetic men [38]. A more recent trial reported the rate of RE among 57 type-1-diabetes mellitus patients (aged 18-50 years) was at least 8.8% [39]. Retrograde ejaculation was also reported in studies of patients who had undergone transurethral resection of prostate (TURP) or open prostatectomy due to disrupted bladder neck integrity. A study of the effect of prostatectomy on QoL in 5,276 men after TURP, found that 68% reported post-surgical RE [40]. However, with the development of less invasive techniques, the incidence of RE decreases following the surgical treatment of LUTS [41–45].
- Painful ejaculation
Painful ejaculation is a common but poorly understood clinical phenomenon, which is associated with sexual dysfunction. Several studies demonstrated its prevalence to range between 1-10% in the general population [46–48]; however, it may increase to 30-75% among men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) [49–53]. It should be noted that the design of most of these studies was not scientifically sound and the condition was probably under-reported due to the lack of an evidence-based definition and well-defined prognostic criteria.
- Haemospermia
The exact incidence and prevalence of haemospermia are difficult to elucidate due to a number of factors including its covert presentation, usually self-limiting nature and patient embarrassment. The symptom represents 1-1.5% of all urological referrals and occurs in all age groups, with a mean age of 37 years [54,55]. In a PCa screening study of 26,126 men, aged > 50 years or older than 40 with a history of PCa or of black ethnicity, haemospermia was found in 0.5% on entry to the trial [56].
- Low sexual desire
The global prevalence of low sexual desire in men is 3-28% [24,57,58]. Low solitary and dyadic sexual desires, have been reported in 68% and 14% of men, respectively [59]. Also, low sexual desire has been observed as a common complaint in gay men, with a prevalence of 19-57% [60,61]. Despite its relationship with age, low sexual desire has been reported among young men (18-29 years), with prevalence of 6-19% [12,62,63].
MANAGEMENT OF ERECTILE DYSFUNCTION:
- Definition and classification
Penile erection is a complex physiological process that involves integration of both neural and vascular events, along with an adequate endocrine milieu. It involves arterial dilation, trabecular smooth muscle relaxation and activation of the corporeal veno-occlusive mechanism [64]. Erectile dysfunction is defined as the persistent inability to attain and maintain an erection sufficient to permit satisfactory sexual performance [65]. Erectile dysfunction may affect psychosocial health and have a significant impact on the QoL of patients and their partner’s [2, 66–68]. There is established evidence that the presence of ED increases the risk of future CV events including myocardial infarction, cerebrovascular events, and all-cause mortality, with a trend towards an increased risk of cardiovascular mortality [69]. Therefore, ED can be an early manifestation of coronary artery and peripheral vascular disease and should not be regarded only as a QoL issue, but also as a potential warning sign of CVD [70–73]. A cost analysis showed that screening men presenting with ED for CVD represents a cost-effective intervention for secondary prevention of both CVD and ED, resulting in substantial cost savings relative to identification of CVD at the time of presentation [74]. Erectile dysfunction is commonly classified into three groups based on aetiology: organic, psychogenic and mixed ED. However, this classification should be used, with caution as most cases are actually of mixed aetiology. It has therefore been suggested to use the terms “primary organic” or “primary psychogenic”.
Risk factors:
Erectile dysfunction is associated with unmodifiable and modifiable common risk factors including age, diabetes mellitus, dyslipidaemia, hypertension, CVD, BMI/obesity/waist circumference, MetS, hyperhomocysteinemia, lack of exercise, and smoking (a positive dose-response association between quantity and duration of smoking has been demonstrated) [67, 71, 75–82]. Furthermore, an association between ED status and pharmaco-therapeutic agents for CVD (e.g., thiazide diuretics and β-blockers, except nebivolol), exert detrimental effects on erectile function, whereas newer drugs (i.e., angiotensin-converting enzymeinhibitors, angiotensin-receptor-blockers and calcium-channel-blockers) have neutral or even beneficial effects [71,83, 84]. Furthermore, the use of psychotropic drugs increases the risk of developing ED [85]. Atrial fibrillation [86], hyperthyroidism [87], vitamin D deficiency [88,89], hyperuricemia [90], folic acid deficiency [91], depression [92] and anxiety disorders [93], chronic kidney disease [81], rheumatic disease [94], stroke [95] and chronic obstructive pulmonary disease [96] have also been reported as risks factors. Available data do not confirm a clear association between ED and hypothyroidism and hyperprolactinaemia [87]. Interestingly, a dual (cause-effect) association between ED and osteoporosis had been proposed, and therefore ED patients should be evaluated by bone mineral density or men with osteoporosis should be further assessed for erectile function [97]. Current evidence supports the fact that renal transplantation improves erectile function and the risk of ED is progressively reduced from before to after surgery [98, 99]. Further epidemiological data have also highlighted other potential risk factors associated with ED including sleep disorders [100], obstructive sleep apnoea [101], psoriasis [102–104], gouty arthritis [100] and ankylosing spondylitis [105], non-alcoholic fatty liver disease [106], other chronic liver disorders [107], chronic periodontitis [108], open-angle glaucoma [109], inflammatory bowel disease [110], chronic fatigue syndrome [111] and allergic rhinitis [112], and spina bifida [113]. Insufficient data are currently available to correlate primarily organic or primarily psychogenic ED with SARS-CoV-2 infection associated disease (COVID-19) [114, 115]. Similarly, although currently available data are scarce, a positive correlation between cycling and ED had been proposed, even if only after adjusting for age and several comorbidities [116]. Recent findings show that pelvic ring fractures are associated with onset of ED with important influence on QoL, especially in young patients [117,118]. A recent meta-analysis showed that men partnered with women suffering from Female Sexual Dysfunction (FSD) present an increased risk of developing sexual impairment, in particular erectile and ejaculatory dysfunction [119]. Erectile dysfunction is also frequently associated with other urological conditions and procedures (Table 1).
Table 1: Urological conditions associated with ED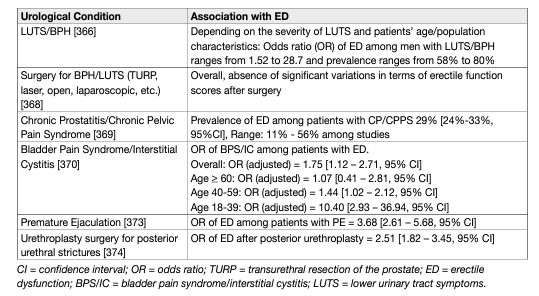
Epidemiological studies have demonstrated consistent evidence for an association between LUTS/BPH and sexual dysfunction, regardless of age, other co-morbidity and lifestyle factors [120]. The Multinational Survey on the Aging Male study, performed in the USA, France, Germany, Italy, Netherlands, Spain, and the UK, systematically investigated the relationship between LUTS and sexual dysfunction in > 12,000 men aged 50-80 years. In the 83% of men who were reported to be sexually active, the overall prevalence of LUTS was 90%, with an overall 49% prevalence of ED and a reported complete absence of erections in 10% of patients. The overall prevalence of ejaculatory disorders was 46% [121]. Regardless of the employed technique, surgery for BPH-LUTS had no significant impact on erectile function at long-term (5 year) follow-up, while a slight advantage is demonstrated for prostate urethral lift (PUL) over conventional TURP at 24 months follow-up [122]. A post-operative improvement of erectile function was even found depending on the degree of LUTS improvement [123, 124]. An association has been confirmed between ED and CP/CPPS [369], and bladder pain syndrome/interstitial cystitis (BPS/IC), mostly in younger men [125]. An association between ED and PE has also been demonstrated [126]. Recent evidence showed that ED is a mild, short-term and transient complication of prostate biopsy, regardless of the trans-rectal or trans-perineal approach employed [127]. An increased risk of ED is reported following [128] open urethroplasty, especially for correction of posterior strictures [129], with recent findings emphasising the importance of patient-reported outcome measures (PROMs) in urethral reconstructive surgery to better report actual sexual function outcomes [130,131].
Several studies have shown that lifestyle modification [132], including physical activity [133], weight loss [134] and pharmacotherapy [84, 135, 136] for CVD risk factors may be of help in improving sexual function in men with ED. Meta-analytic data reveals a positive effect of lipid-lowering therapy with statins on erectile function [137, 138]. However, it should be emphasised that further controlled prospective studies are necessary to determine the effects of exercise or other lifestyle changes in the prevention and treatment of ED [132].
Pathophysiology:
The pathophysiology of ED may be vasculogenic, neurogenic, anatomical, hormonal, drug-induced and/or psychogenic (Table 2) [64].
In most cases, numerous pathophysiological pathways can co-exist and may all negatively impact on erectile function.
The proposed ED etiological and pathophysiological division should not be considered prescriptive. In most cases, ED is associated with more than one pathophysiological factor and very often, if not always, will have a psychological component. Likewise, organic components can negatively affect erectile function with different pathophysiological effects. Therefore, Table 2 must be considered for diagnostic classifications only (along with associated risk factors for each subcategory).
Table 2: Pathophysiology of ED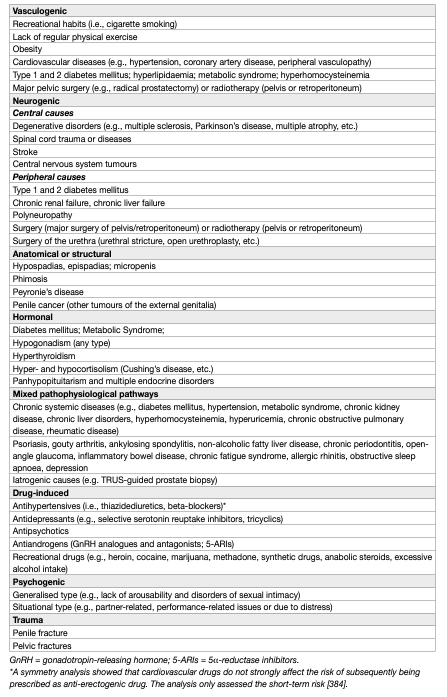
Pelvic surgery and prostate cancer treatment:
Pelvic surgery, especially for oncological disease (e.g., radical prostatectomy (RP) [139] or radical cystectomy [140] and colorectal surgery [141]), may have a negative impact on erectile function and overall sexual health. The most relevant causal factor is a lesion occurring in the neurovascular bundles that control the complex mechanism of the cavernous erectile response, whose preservation (either partial or complete) during surgery eventually configures the so-called nerve-sparing (NS) approach [142]. Therefore, surgery resulting in damage of the neurovascular bundles, results in ED, although NS approaches have been adopted over the last few decades. This approach is applicable to all types of surgery that are potentially harmful to erectile function, although to date, only the surgical treatment of PCa has enough scientific evidence supporting its potential pathophysiological association with ED [143,144]. However, even non-surgical treatments of PCa (i.e., radiotherapy, or brachytherapy) can be associated with ED [145, 146]. The concept of active surveillance for the treatment of PCa was developed to avoid over-treatment of non-significant localised low-risk diseases, while limiting potential functional adverse effects (including ED). However, it is interesting that data suggest that even active surveillance has a detrimental impact on erectile function (and sexual well-being as a whole) [147–149].
Radical prostatectomy (open, laparoscopic or robot-assisted) is a widely performed procedure with a curative intent for patients presenting with clinically localised intermediate- or high-risk PCa and a life expectancy of > 10 years based on health status and co-morbidity [150]. This procedure may lead to treatment-specific sequelae affecting health-related QoL. Men undergoing RP (any technique) should be adequately informed before the operation that there is a significant risk of sexual changes other than ED, including decreased libido, changes in orgasm, anejaculation, Peyronie’s-like disease, and changes in penile length [144, 146]. These outcomes have become increasingly important with the more frequent diagnosis of PCa in both younger and older men [151, 152]. Research has shown that 25-75% of men experience post-RP ED [153], even though these findings had methodological flaws; in particular, the heterogeneity of reporting and assessment of ED among the studies [143, 154]. Conversely, the rate of unassisted post-operative erectile function recovery ranged between 20 and 25% in most studies. These rates have not substantially improved or changed over the past 17 years, despite growing attention to post-surgical rehabilitation protocols and refinement of surgical techniques [154–156]. Overall, patient age, baseline erectile function and surgical volume, with the consequent ability to preserve the neurovascular bundles, seem to be the main factors in promoting the highest rates of post-operative potency [144, 151, 153, 157]. Regardless of the surgical technique, surgeons’ experience may clearly impact on postoperative EF outcome; in particular when surgeons have a caseload greater than 25 radical prostatectomy cases per year or total cumulative experience of >1,000 prostatectomy cases result in better erectile function outcomes after RP [158]. Patients being considered for nerve-sparing RP (NSRP) should ideally be potent pre-operatively [151]. The recovery time following surgery is of clinical importance in terms of post-operative recovery of erectile function. Available data confirm that post-operative erectile function recovery can occur up to 48 months after RP [159]. Likewise, it has been suggested that post-operative therapy (any type) should be commenced as soon as possible after the surgical procedure [151, 153], although evidence suggests that the number of patients reporting return of spontaneous erectile function has not increased. In terms of the effects of surgical interventions (e.g., robot-assisted RP [RARP] vs. other types of surgery), data are still conflicting. An early systematic review showed a significant advantage in favour of RARP in comparison with open retropubic RP in terms of 12-month potency rates [160], without significant differences between laparoscopic RP and RARP. Some recent reports confirm that the probability of erectile function recovery is about twice as high for RARP compared with open RP [161]. More recently, a prospective, controlled, nonrandomised trial of patients undergoing RP in 14 Swedish centres comparing RARP versus open retropubic RP, showed a small improvement in erectile function after RARP [162]. Conversely, a randomised controlled phase 3 study of men assigned to open RP or RARP showed that the two techniques yielded similar functional outcomes at 12 weeks [163]. More controlled prospective well-designed studies, with longer follow-up, are necessary to determine if RARP is superior to open RP in terms of post-operative ED rates [164]. To overcome the problem of heterogeneity in the assessment of erectile function, for which there is variability in terms of the PROMs used (e.g., International Index of Erectile Function [IIEF], IIEF-5, Expanded Prostate Cancer Index Composite with 26 items [EPIC 26], Sexual Health Inventory for Men, etc.) to measure potency or erectile function, the criteria used to define restoration of erectile function should be re-evaluated utilising objective and validated thresholds (e.g., normalisation of scores or return to baseline erectile function) [143]. Erectile dysfunction is also a common problem after both external beam radiation therapy (EBRT) and brachytherapy for PCa. A systematic review and meta-analysis including men treated with EBRT (65%), brachytherapy (31%) or both (4%) showed that the post-treatment prevalence of ED was 34% at 1 year and 57% at 5.5 years [165, 166]. Similar findings have been reported for stereotactic radiotherapy with 26-55% of previously sexually functioning patients reporting ED at 5 years [165]. Recently other modalities have emerged as potential therapeutic options in patients with clinically-localised PCa, including whole gland and focal (lesion-targeted) treatments, to ablate tumours selectively while limiting sexual toxicity by sparing the neurovascular bundles. These include high-intensity focused US (HIFU), cryotherapeutic ablation of the prostate (cryotherapy), focal padeliporfin-based vascular-targeted photodynamic therapy and focal radiation therapy (RT) by brachytherapy or CyberKnife®. All these approaches have a less negative impact on erectile function with many studies reporting a complete recovery at one-year followup [166]. However, prospective randomised controlled studies are needed to compare the functional and oncological outcomes using different treatment modalities [167, 168].
Diagnostic evaluation (basic work-up):
- Medical and sexual history
The first step in evaluating ED is always a detailed medical and sexual history of patients and, when available, their partners [169]. It is important to establish a relaxed atmosphere during history-taking. This will make it easier to ask questions about erectile function and other aspects of the patient’s sexual history; and to explain the diagnosis and therapeutic approach to the patient and their partner. Figure 1 lists the minimal diagnostic evaluation (basic work-up) in patients with ED.
Figure 1: Minimal diagnostic evaluation (basic work-up) in patients with ED
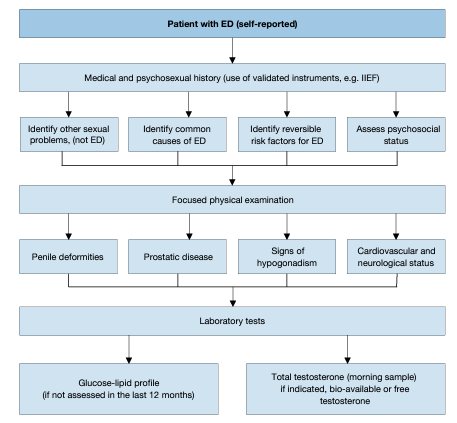
The sexual history must include information about previous and current sexual relationships, current emotional status, onset and duration of the erectile problem, and previous consultations and treatments. The sexual health status of the partner(s) (when available) can also be useful. A detailed description should be made of the rigidity and duration of both sexually-stimulated and morning erections and of problems with sexual desire, arousal, ejaculation, and orgasm [170, 171]. Validated psychometric questionnaires, such as the IIEF [172] or its short version (i.e., Sexual Health Inventory for Men; SHIM) [172], help to assess the different sexual function domains (i.e. sexual desire, erectile function, orgasmic function, intercourse satisfaction, and overall satisfaction), as well as the potential impact of a specific treatment modality. Similarly, structured interviews allow the identification and quantification of the different underlying factors affecting erectile function [173]. Psychometric analyses also support the use of the Erectile Hardness Score (EHS) for the assessment of penile rigidity in practice and in clinical trials research [174]. In cases of depressive mood, clinicians may use the Beck Depressive Inventory [175], which is one of the most recognised self-reported measures in the field, takes approximately 10 minutes to complete, and assigns the patient to a specific level of depression (varying from “normal mood” to “extreme depression”). Patients should always be screened for symptoms of possible hypogonadism (testosterone deficiency), including decreased energy and libido, and fatigue; potential cognitive impairment may be also observed in association with hypogonadism, as well as for LUTS. In this regard, although LUTS/BPH in themselves do not represent contraindications to treatment for LOH, screening for LUTS severity is clinically relevant [176].
- Physical examination
Every patient must be given a physical examination focused on the genitourinary, endocrine, vascular and neurological systems [177, 178]. A physical examination may reveal unsuspected diagnoses, such as Peyronie’s disease, pre-malignant or malignant genital lesions, prostatic enlargement or irregularity/nodularity, or signs and symptoms suggestive of hypogonadism (e.g., small testes or alterations in secondary sexual characteristics). Assessment of previous or concomitant penile abnormalities (e.g., hypospadias, congenital curvature, or Peyronie’s disease with preserved rigidity) during the medical history and the physical examination is mandatory. Blood pressure and heart rate should be measured if they have not been assessed in the previous 3-6 months. Likewise, either BMI calculation or waist circumference measurement should be undertaken to assess patients for comorbid conditions (e.g., MetS).
- Laboratory testing
Laboratory testing must be tailored to the patient’s complaints and risk factors. Patients should undergo a fasting blood glucose or haemoglobin A1c and lipid profile measurement if they have not been assessed in the previous 12 months. Hormonal tests should include early morning total testosterone in a fasting state. The bioavailable or calculated-free testosterone values may sometimes be needed to corroborate total testosterone measurements. However, the threshold of testosterone required to maintain an erection is low and ED is usually a symptom of more severe cases of hypogonadism [87, 179, 180–182]. Additional laboratory tests may be considered in selected patients with specific signs and associated symptoms (e.g., PSA) [183], prolactin and luteinising hormone [184]). Although physical examination and laboratory evaluation of most men with ED may not reveal the exact diagnosis, clinical and biochemical evaluation presents an opportunity to identify comorbid conditions [178].
- Cardiovascular system and sexual activity: the patient at risk
Patients who seek treatment for sexual dysfunction have a high prevalence of CVDs. Epidemiological surveys have emphasised the association between cardiovascular/metabolic risk factors and sexual dysfunction in both men and women [185]. Overall, ED can improve the sensitivity of screening for asymptomatic CVD in men with diabetes [186, 187]. Erectile dysfunction significantly increases the risk of CVD, coronary heart disease and stroke. Furthermore, the results of a recent prospective cohort study showed that ED is an independent predictor for incident atrial fibrillation [188]. All of this cause mortality and the increase is probably independent of conventional cardiovascular risk factors [70, 71, 189, 190]. Longitudinal data from an observational population-based study of 965 men without CVD showed that younger men (especially those < 50 years) with transient and persistent ED have an increased Framingham CVD risk [191]. The EAU Guidelines for diagnosing and treating men with ED have been adapted from previously published recommendations from the Princeton Consensus conferences on sexual dysfunction and cardiac risk [192]. The Princeton Consensus Conference is dedicated to optimising sexual function and preserving cardiovascular health [192-194]. Accordingly, patients with ED can be stratified into three cardiovascular risk categories (Table 3), which can be used as the basis for a treatment algorithm for initiating or resuming sexual activity (Figure 2). It is also possible for the clinician to estimate the risk of sexual activity in most patients from their level of exercise tolerance, which can be determined when taking the patient’s history [136].
Table 3: Cardiac risk stratification (based on 2nd and 3rd Princeton Consensus)
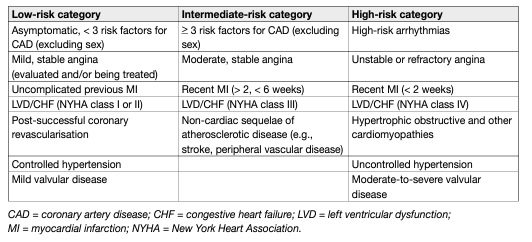
Figure 2: Treatment algorithm for determining level of sexual activity according to cardiac risk in ED (based on 3rd Princeton Consensus)
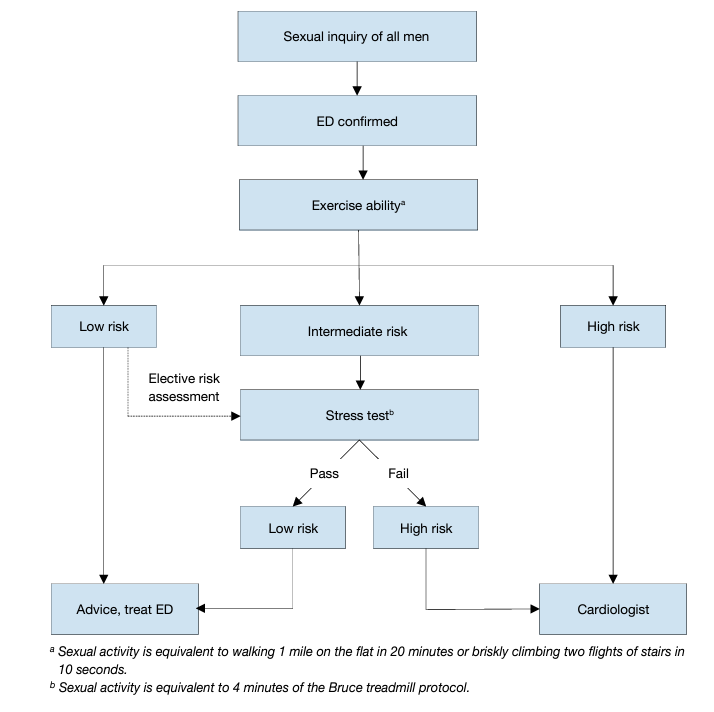
- Low-risk category
The low-risk category includes patients who do not have any significant cardiac risk associated with sexual activity. Low-risk is typically implied by the ability to perform exercise of modest intensity, which is defined as, > 6 metabolic equivalents of energy expenditure in the resting state, without symptoms. According to current knowledge of the exercise demand or emotional stress associated with sexual activity, low-risk patients do not need cardiac testing or evaluation before initiation or resumption of sexual activity or therapy for sexual dysfunction.
- Intermediate- or indeterminate-risk category
The intermediate- or indeterminate-risk category consists of patients with an uncertain cardiac condition or patients whose risk profile requires testing or evaluation before the resumption of sexual activity. Based upon the results of testing, these patients may be moved to either the high- or low-risk group. A cardiology consultation may be needed in some patients to help the primary physician determine the safety of sexual activity.
- High-risk category
High-risk patients have a cardiac condition that is sufficiently severe and/or unstable for sexual activity to carry a significant risk. Most high-risk patients have moderate-to-severe symptomatic heart disease. High-risk individuals should be referred for cardiac assessment and treatment. Sexual activity should be stopped until the patient’s cardiac condition has been stabilised by treatment, or a decision made by the cardiologist and/or internist that it is safe to resume sexual activity.
- Diagnostic Evaluation (advanced work-up)
Most patients with ED can be managed based on the basis of medical and sexual history; conversely, some patients may need specific diagnostic tests (Tables 4 and 5).
Table 4: Indications for specific diagnostic tests for ED

Table 5: Specific diagnostic tests for ED

- Nocturnal penile tumescence and rigidity test
The nocturnal penile tumescence and rigidity (NPTR) test applies nocturnal monitoring devices that measure the number of erectile episodes, tumescence (circumference change by strain gauges), maximal penile rigidity, and duration of nocturnal erections. The NPTR assessment should be performed on at least two separate nights. A functional erectile mechanism is indicated by an erectile event of at least 60% rigidity recorded on the tip of the penis that lasts for > 10 minutes [195]. Nocturnal penile tumescence and rigidity monitoring is an attractive approach for objectively differentiating between organic and psychogenic ED (patients with psychogenic ED usually have normal findings in the NPTR test). However, many potential confounding factors (e.g., situational) may limit its routine use for diagnostic purposes [196].
- Intracavernous injection test
The intracavernous injection test gives limited information about vascular status. A positive test is a rigid erectile response (unable to bend the penis) that appears within 10 minutes after the intracavernous injection and lasts for 30 minutes [197]. Overall, the test is inconclusive as a diagnostic procedure and a duplex Doppler study of the penis should be requested, if clinically warranted.
- Dynamic duplex ultrasound of the penis
Dynamic duplex ultrasound (US) of the penis is a second-level diagnostic test that specifically studies the haemodynamic pathophysiology of erectile function. Therefore, in clinical practice, it is usually applied in those conditions in which a potential vasculogenic aetiology of ED (e.g., diabetes mellitus, renal transplantation, multiple concomitant CV risk factors and/or overt peripheral vascular disease, and poor responders to oral therapy) is suspected. Peak systolic blood flow > 30 cm/s, end-diastolic velocity < 3 cm/s and resistance index > 0.8 are considered normal [198, 199]. Recent data suggest that duplex scanning as a haemodynamic study may be better at tailoring therapy for ED, such as for low-intensity shock wave treatment (LI-SWT) and for diagnosing vasculogenic ED [200]. Further vascular investigation is unnecessary if a duplex US examination is normal.
- Psychopathological and psychosocial assessment
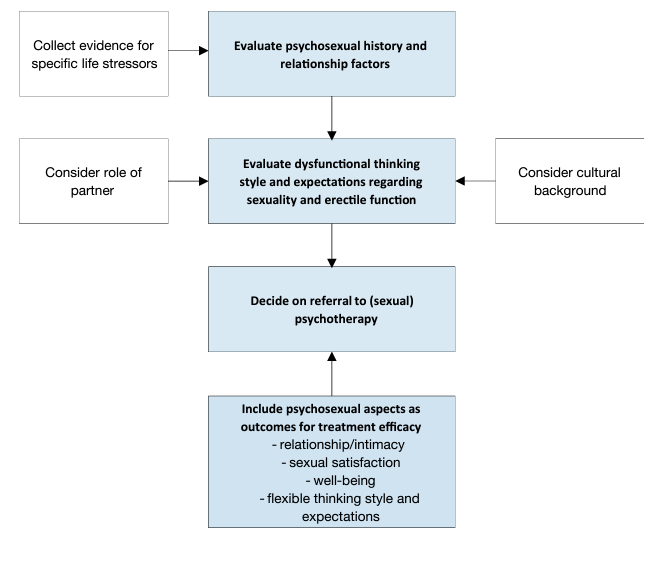
Mental health issues and psychological distress are frequently comorbid with ED (figure 3) [201]. This is most evident for depression and anxiety related disorders, but may also include transitory states of altered mood (i.e., dysfunctional affective states resulting from a specific life stressor or crisis) [92, 202, 203]. Relationship factors, including lack of satisfaction with the partner, poor sexual relationships, length of the relationship, or feeling emotionally disconnected from the partner during sex, have been related to erectile difficulties and dysfunction [202, 204, 205]. In contrast, intimacy was found to be a protective factor in ED [82, 206]. Additionally, the cognitive factors underpinning organic and non-organic ED must also be assessed. Cognitive factors include male dysfunctional thinking styles and expectations about sexuality and sexual performance. These expectations result from the sexuality norms and stereotypes, shared by a given culture. Expectations emphasising high sexual performance in men, result in anxiety, which acts as a maintenance factor for ED [207, 208]. Unrealistic expectations about male sexual performance may further align with internal causal attributions regarding the loss of erection (i.e., men attribute the loss of erection to themselves [sense of personal inadequacy]), thereby worsening ED [207,209]. Likewise, poor self-esteem and cognitive distraction from erotic cues, are expected to negatively affect ED [210, 211].
Figure 3: Psychopatholgical and psychosocial assessment
Treatment of erectile dysfunction:
- Patient education – consultation and referrals
Educational intervention is often the first approach to sexual complaints, and consists of informing patients about the psychological and physiological processes involved in the individual’s sexual response, in ways the patient can understand. This first level approach has been shown to favour sexual satisfaction in men with ED [212]. Accordingly, consultation with the patient should include a discussion of the expectations and needs of the patient’s and their sexual partner. It should also review the patient’s and partner’s understanding of ED and the results of diagnostic tests, and provide a rationale for treatment selection [213]. Patient and partner education is an essential part of ED management [213, 214], and may prevent misleading information that can be at the core of dysfunctional psychological processes underpinning ED.
- Treatment options
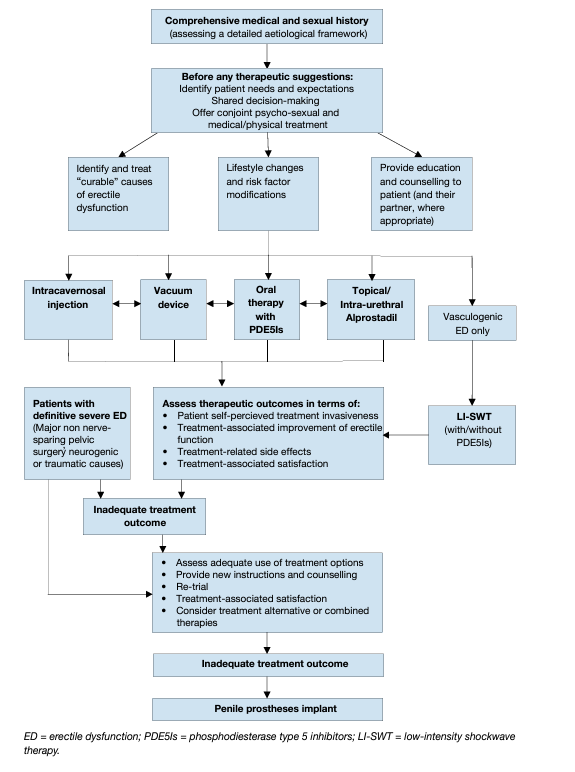
Based on the currently available evidence, a novel comprehensive therapeutic and decision-making algorithm (Figure 4) for treating ED, which takes into account the level of invasiveness of each therapy and its efficacy, has been presented. This newly-developed treatment algorithm was extensively discussed within the guidelines as an alternative to the traditional three-level concept, to better tailor a personalised therapy to individual patients, according to invasiveness, tolerability and effectiveness of the different therapeutic options and patients’ expectations. In this context, patients should be fully counselled with respect to all available treatment modalities.
Erectile dysfunction may be associated with modifiable or reversible risk factors, including lifestyle or drugrelated factors [132]. These factors may be modified either before, or at the same time as, specific therapies are used. Likewise, ED may be associated with concomitant and underlying conditions (e.g., endocrine disorders and metabolic disorders such as diabetes, and some cardiovascular problems such as hypertension) which should always be well-controlled as the first step of any ED treatment [215]. Major clinical potential benefits of lifestyle changes may be achieved in men with specific co-morbid CV or metabolic disorders, such as diabetes or hypertension [132, 216].
As a rule, ED can be treated successfully with current treatment options, but it cannot be cured. The only exceptions are psychogenic ED, post-traumatic arteriogenic ED in young patients, and hormonal causes (e.g., hypogonadism) [217, 184], which potentially can be cured with specific treatments. Most men with ED are not treated with cause-specific therapeutic options. This results in a tailored treatment strategy that depends on invasiveness, efficacy, safety and cost, as well as patient preference [213]. In this context, physician-patient (partner, if available) dialogue is essential throughout the management of ED. Interesting insights come from a recent systematic review that showed a consistent discontinuation rate for all available treatment options (4.4-76% for PDE5Is); 18.6-79.9% for intracavernous injections; 32-69.2% for urethral suppositories; and 30% for penile prostheses). Men’s beliefs about ED treatment, therapeutic ineffectiveness, adverse effects, quality of men’s intimate relationships and treatment costs are the most prevalent barriers to treatment actual use [218].
Figure 4: Management algorithm for erectile dysfunction
- Oral pharmacotherapy
Four potent selective PDE5Is have been approved by the EMA for treatment of ED [219]. Phosphodiesterase type 5 catalyses the hydrolysis of the second messenger cyclic guanosine monophosphate (cGMP) in the cavernous tissue; cGMP is involved in intra-cellular signalling pathways of cavernous smooth muscle. Accumulation of cGMP sets in motion a cascade of events at the intracellular level, which induces a loss of contractile tone of the penile vessels by lowering cytosolic Ca2+. Nitric oxide (NO) has an essential role in promoting the formation of cGMP and other pathways leading to corporeal smooth muscle relaxation and erection of the penis [215, 220]. This is associated with increased arterial blood flow, eventually leading to compression of the sub-tunical venous plexus followed by erection [221]. Since they are not initiators of erection, PDE5Is require sexual stimulation to facilitate an erection. Efficacy is defined as an erection, with rigidity, sufficient for satisfactory intercourse [215].
- Sildenafil
Sildenafil was launched in 1998 and was the first PDE5I available on the market [222]. It is administered in doses of 25, 50 and 100 mg. The recommended starting dose is 50 mg and should be adapted according to the patient’s response and adverse effects [222]. Sildenafil is effective 30-60 minutes after administration [222]. Its efficacy is reduced after a heavy, fatty meal due to delayed absorption. Efficacy may be maintained for up to 12 hours [223]. The pharmacokinetic profile for sildenafil is presented in Table 6. Adverse events (Table7) are generally mild in nature and self-limited [224, 225]. After 24 weeks in a dose-response study, improved erections were reported by 56%, 77% and 84% in a general ED population taking 25, 50 and 100 mg sildenafil, respectively, compared to 25% of men taking placebo [226]. Sildenafil significantly improved patient scores for IIEF, sexual encounter profile question 2 (SEP2), SEP question 3 (SEP3) and General Assessment Questionnaire (GAQ) and treatment satisfaction. The efficacy of sildenafil in almost every subgroup of patients with ED has been successfully established, irrespective of age [227]. Recently, an orally disintegrating tablet (ODT) of sildenafil citrate at a dose of 50 mg has been developed, mainly for patients who have difficulty swallowing solid dosage forms.
- Tadalafil
Tadalafil was licensed for treatment of ED in February 2003 and is effective from 30 minutes after administration, with peak efficacy after about 2 hours [228]. Efficacy is maintained for up to 36 hours [228] and is not affected by food [229]. Usually, tadalafil is administered in on-demand doses of 10 and 20 mg or a daily dose of 5 mg. The recommended on-demand starting dose is 10 mg and should be adapted according to the patient’s response and adverse effects [228, 230]. Pharmacokinetic data for tadalafil are presented in Table 6. Adverse effects (Table 7) are generally mild in nature and self-limited by continuous use. In pre-marketing studies, after 12 weeks of treatment in a dose-response study, improved erections were reported by 67% and 81% of men with ED taking 10 and 20 mg tadalafil, respectively, compared to 35% of men in the placebo control group [228]. Tadalafil significantly improves patient scores for IIEF, SEP2, SEP3, and GAQ and treatment satisfaction [228]. Efficacy of tadalafil has been confirmed in post-marketing studies [219, 231] and in almost every subgroup of patients with ED, including difficult-to-treat subgroups (e.g., diabetes mellitus) [232]. Tadalafil has also been shown to have a net clinical benefit in the short-term on ejaculatory and orgasmic functions in ED patients [233]. Daily tadalafil 5 mg has also been approved and licensed as a monotherapy in men with BPH related LUTS, due to its ability to significantly improve urinary symptoms [237]. Therefore, its use may be considered in patients with ED patients also complaining of concomitant LUTS, and wishing to benefit from a single therapy [238]. Data have confirmed that 40% of men aged > 45 years were combined responders for ED and LUTS/BPH to treatment with tadalafil 5 mg once daily, with symptom improvement after 12 weeks [239]. To date, the main limitation on the use of daily tadalafil 5 mg as a combined therapy for both ED and LUTS is conditioned by the fact that no data are available in terms of efficacy and tolerability beyond 12 months of treatment.
- Vardenafil
Vardenafil became commercially available in March 2003 and is effective from 30 minutes after administration [240], with one of three patients achieving satisfactory erections within 15 minutes of ingestion [241]. Its effect is reduced by a heavy, fatty meal. Doses of 5, 10 and 20 mg have been approved for on-demand treatment of ED. The recommended starting dose is 10 mg and should be adapted according to the patient’s response and adverse effects [242]. Pharmacokinetic data for vardenafil are presented in Table 6. Adverse events (Table 7) are generally mild in nature and self-limited by continuous use [242]. After 12 weeks in a dose-response study, improved erections were reported by 66%, 76% and 80% of men with ED taking 5, 10 and 20 mg vardenafil, respectively, compared with 30% of men taking placebo [242, 243]. Vardenafil significantly improved patient scores for IIEF, SEP2, SEP3, and GAQ and treatment satisfaction. Efficacy has been confirmed in post-marketing studies [242, 243]. The efficacy of vardenafil in almost every subgroup of patients with ED, including difficult-to-treat subgroups (e.g. diabetes mellitus), has been successfully established. An orodispersable tablet (ODT) formulation of vardenafil has been released [243]. Orodispersable tablet formulations offer improved convenience over film-coated formulations and may be preferred by patients. Absorption is unrelated to food intake and they exhibit better bio-availability compared to film-coated tablets [244]. The efficacy of vardenafil ODT has been demonstrated in several RCTs and did not seem to differ from the regular formulation [244–246].
- Avanafil
Avanafil is a highly-selective PDE5I that became commercially available in 2013 [247]. Avanafil has a high ratio of inhibiting PDE5 as compared with other PDE subtypes, ideally allowing for the drug to be used for ED while minimising adverse effects (although head-to-head comparisons are not yet available) [248]. Doses of 50, 100 and 200 mg have been approved for on-demand treatment of ED [247]. The recommended starting dose is 100 mg taken as needed 15-30 minutes before sexual activity and the dose may be adapted according to efficacy and tolerability [247, 249, 250]. In the general population with ED, the mean percentage of attempts resulting in successful intercourse was approximately 47%, 58% and 59% for the 50, 100 and 200 mg groups, respectively, as compared with ~28% for placebo [247, 249]. Data from sexual attempts made within 15 minutes of treatment showed successful attempts in 64%, 67% and 71% of cases treated with avanafil 50, 100 and 200 mg, respectively. Dose adjustments are not warranted based on renal function, hepatic function, age or sex [249]. Pharmacokinetic data for avanafil are presented in Table 6 [247, 249]. Adverse effects are generally mild in nature (Table 7) [247, 249]. Pairwise meta-analytic data from available studies have suggested that avanafil significantly improved patient scores for IIEF, SEP2, SEP3 and GAQ, with an evident dose-response relationship [247, 251]. Administration with food may delay the onset of effect compared with administration in a fasting state but avanafil can be taken with or without food [252]. The efficacy of avanafil in many groups of patients with ED, including difficult-to-treat subgroups (e.g., diabetes mellitus), has been successfully established. As for dosing, 36.4% (28 of 77) of sexual attempts (SEP3) at < 15 minutes were successful with avanafil vs. 4.5% (2 of 44) after placebo (P < 0.01) [253]. A recent meta-analysis confirmed that avanafil had comparable efficacy with sildenafil, vardenafil and tadalafil [252].
- Choice or preference among the different PDE5Is
To date, no data are available from double- or triple-blind multicentre studies comparing the efficacy and/ or patient preference for the most-widely available PDE5Is (i.e., sildenafil, tadalafil, vardenafil, and avanafil). Choice of drug depends on frequency of intercourse (occasional use or regular therapy, 3-4 times weekly) and the patient’s personal experience. Patients need to know whether a drug is short- or long-acting, its possible disadvantages, and how to use it. Two different network meta-analyses demonstrated that ED patients who prioritise high efficacy must use sildenafil 50 mg whereas those who optimise tolerability should initially use tadalafil 10 mg and switch to Udenafil 100 mg if the treatment is not sufficient (however, Udenafil 100 mg is not EMA or US Food and Drug Administration approved and is not available in Europe) [231, 254]. The results of another clinical trial have revealed that tadalafil 5 mg once daily may improve erectile function among men who have a partial response to on-demand PDE5I therapy [255].
- Continuous use of PDE5Is
From a pathophysiological standpoint, animal studies have shown that chronic use of PDE5Is significantly improves or prevents the intracavernous structural alterations caused by age, diabetes or surgical damage [256–260]. No data exist in humans. In humans, a RCT has shown that there is no clinically beneficial effect on endothelial dysfunction measured by flow-mediated dilation deriving from daily tadalafil when compared to placebo [261]. From a clinical standpoint, in 2007, tadalafil 2.5 and 5 mg/day were approved by the EMA for treatment of ED. According to the EMA, a once-daily regimen with tadalafil 2.5 or 5 mg might be considered suitable, based on patients’ choice and physicians’ judgement. In these patients, the recommended dose is 5 mg, taken once daily at approximately the same time. Overall, tadalafil, 5 mg once daily, provides an alternative to on-demand tadalafil for couples who prefer spontaneous rather than scheduled sexual activities or who anticipate frequent sexual activity, with the advantage that dosing and sexual activity no longer need to be linked. Overall, treatment with tadalafil 5 mg once daily in men complaining of ED of various severities is well-tolerated and effective [262]. An integrated analysis showed that, regardless of the type of ED population, there is no clinically significant difference between a tadalafil treatment administered with continuous (once daily) vs. on-demand regimen [261]. The appropriateness of the continuous use of a daily regimen should be re-assessed periodically [262, 263].
Safety issues for PDE5Is:
(i) Cardiovascular safety
Clinical trial results for the four PDE5Is and post-marketing data of sildenafil, tadalafil, and vardenafil have demonstrated no increase in myocardial infarction rates in patients receiving PDE5Is, as part of either RCTs or open-label studies, or compared to expected rates in age-matched male populations. None of the PDE5Is has an adverse effect on total exercise time or time-to-ischaemia during exercise testing in men with stable angina [219, 264]. Chronic or on-demand use is well-tolerated with a similar safety profile.
(ii) Contraindication for the concomitant use of organic nitrates
An absolute contraindication to PDE5Is is use of any form of organic nitrate (e.g., nitroglycerine, isosorbide mononitrate, and isosorbide dinitrate) or NO donors (e.g., other nitrate preparations used to treat angina, as well as amyl nitrite or amyl nitrate such as “poppers” that are used for recreation). They result in cGMP accumulation and unpredictable falls in blood pressure and symptoms of hypotension. The duration of interaction between organic nitrates and PDE5Is depends upon the PDE5I and nitrate used. If a PDE5I is taken and the patient develops chest pain, nitroglycerine must be withheld for at least 24 hours if sildenafil (and probably also vardenafil) is used (half-life, 4 hours), or at least 48 hours if tadalafil is used (half-life, 17.5 hours), and for no less than 12 hours if avanafil is used (half-life, 6-17 hours) [265–268].
(iii) Use caution with antihypertensive drugs
Co-administration of PDE5Is with antihypertensive agents (e.g., angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, calcium blockers, β-blockers, and diuretics) may result in small additive decreases in blood pressure, which are usually minor [192]. In general, the adverse event profile of a PDE5I is not worsened by a background of antihypertensive medication, even when the patient is taking several antihypertensive agents [269].
(iv) Interaction with Nicorandil
In vitro studies in animals suggest that the potassium channel opener nicorandil may potentiate the vasorelaxation induced by isoproterenol in isolated rat aorta by increasing cyclic GMP levels [270]. This may be due to the nitric oxide donating properties of nicorandil. Therefore, concurrent use of nicorandil and PDE5Is is also contraindicated.
- α-Blocker interactions
Tadalafil 5 mg is currently the only licensed drug for the treatment of both ED and LUTS with level 1 evidence confirming its overall good efficacy in relieving urinary symptoms and improving erectile function [238]. As such, this treatment should be considered in patients suffering from mild to moderate LUTS associated with ED either alone or in combination with alpha-blockers. To this regard, given that both drugs are vasodilators with a potential risk of hypotension, historically there has always been caution in the combination of alpha-blockers and PDE5I (any) because of the fear of possible cumulative effects on blood pressure, based on the evidence from some individual studies that reported the tolerability of combination therapy [223, 241, 271]. However, a recent meta-analysis concluded that a concomitant treatment with α-blockers [both non-uroselective (e.g., terazosin and doxazosin) and uro-selective (e.g., alfuzosin, tamsulosin and silodosin) and PDE5Is may produce changes in haemodynamic parameters, but it does not increase the rate of adverse events due to hypotension [270]. Therefore, there is no current limitation in the simultaneous use of α-blockers and PDE5I, prioritising the use of uro-selective drugs in order to further minimise the risk of dizziness or other adverse events, thus including hypotension.
- Dosage adjustment
Drugs that inhibit the CYP34A pathway inhibit the metabolic breakdown of PDE5Is, thus increasing PDE5Is blood levels (e.g., ketoconazole, ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir and telithromycin). Therefore, lower doses of PDE5Is are necessary. However, other agents, such as rifampin, phenobarbital, phenytoin and carbamazepine, may induce CYP3A4 and enhance the breakdown of PDE5Is, so that higher doses of PDE5Is are required. Severe kidney or hepatic dysfunction may require dose adjustments or warnings.
- Management of non-responders to PDE5Is
The two main reasons why patients fail to respond to a PDE5I are either incorrect drug use or lack of efficacy. Data suggest that an adequate trial involves at least six attempts with a particular drug [272]. The management of non-responders depends upon identifying the underlying cause [273]. Check that the medication has been properly prescribed and correctly used. The main reason why patients fail to use their medication correctly is inadequate counselling from their physician. The most common causes of incorrect drug use are: i) failure to use adequate sexual stimulation; ii) failure to use an adequate dose; and, iii) failure to wait an adequate amount of time between taking the medication and attempting sexual intercourse. Check that the patient has been using a licensed medication. There is a large counterfeit market in PDE5Is. The amount of active drug in these medications varies enormously and it is important to check how and from which source the patient has obtained his medication. PDE5I action is dependent on the release of NO by the parasympathetic nerve endings in the erectile tissue of the penis. The usual stimulus for NO release is sexual stimulation, and without adequate sexual stimulation (and NO release), the medication is ineffective. Furthermore, the reduced production of NO that occurs in diabetic patients due to peripheral neuropathy, is thought to be the justification for the higher failure rate of PDE5Is in this category of patients. Oral PDE5Is take different times to reach maximal plasma concentrations (Cmax) [223, 225, 244, 251, 274–276]. Although pharmacological activity is achieved at plasma levels below the maximal plasma concentration, there will be a period of time following oral ingestion of the medication during which the drug is ineffective. Even though all four drugs have an onset of action in some patients within 15-30 minutes of oral ingestion [225, 244, 274–276], most patients require a longer delay between taking the medication [242, 251, 277, 278]. Absorption of both sildenafil and vardenafil can be delayed by a heavy, fatty meal [279]. Absorption of tadalafil is less affected, and food has negligible effects on its bioavailability [274]. When avanafil is taken with a high-fat meal, the rate of absorption is reduced with a mean delay in Tmax of 1.25 hours and a mean reduction in Cmax of 39% (200 mg). There is no effect on the extent of exposure (area under the curve). The small changes in avanafil Cmax are considered to be of minimal clinical significance [247, 248, 251]. It is possible to wait too long after taking the medication before attempting sexual intercourse. The half-life of sildenafil and vardenafil is ~4 hours, suggesting that the normal window of efficacy is 6-8 hours following drug ingestion, although responses following this time period are recognised. The half-life of avanafil is 6-17 hours. Tadalafil has a longer half-life of ~17.5 hours, so the window of efficacy is longer at ~36 hours. Data from uncontrolled studies suggest patient education can help salvage an apparent non-responder to a PDE5I [273, 280–283]. After emphasising the importance of dose, timing, and sexual stimulation to the patient, erectile function can be effectively restored following re-administration of the relevant PDE5I [273, 280, 281].
A systematic review has addressed the association between genetic polymorphism, especially those encoding endothelial nitric oxide synthase, and the variability in response to PDE5Is [284]. Similar recent data have suggested that response to sildenafil treatment is also dependent on polymorphism in the PDE5A gene, which encodes the principal cGMP-catalysing enzyme in the penis, regulating cGMP clearance, and it is the primary target of sildenafil [285–287].
- Clinical strategies in patients correctly using a PDE5Is
Overall, treatment goals should be individualised to restore sexual satisfaction for patients and/or couples, and improve QoL based on patients expressed needs and desires [288]. In this context, data suggests that almost half of patients abandon first-generation PDE5Is within 1 year, with no single specific factor playing a major role in dropout rates [289]. Uncontrolled trials have demonstrated that hypogonadal patients not responding to PDE5Is may improve their response to PDE5Is after initiating testosterone therapy [217, 215, 290]. Therefore, in the real-life setting most patients with ED will first be prescribed a PDE5I, which is usually effective; however, if diagnostic criteria suggestive for testosterone deficiency are present, testosterone therapy may be more appropriate even in ED patients [291, 217]. Modification of other risk factors may also be beneficial, as previously discussed. Limited data suggest that some patients might respond better to one PDE5I than to another [292], and although these differences might be explained by variations in drug pharmacokinetics, they do raise the possibility that, despite an identical mode of action, switching to a different PDE5I might be helpful. However, it is important to emphasise that the few randomised studies have shown any difference in clinical outcomes with different drugs and intake patterns in patients with classic ED [293] and in special populations such as people with diabetics [294]. In refractory, complex, or difficult-to-treat cases of ED patients a combination therapy should be considered as a first-line approach. Although the available data are still limited, combining PDE5I with antioxidant agents, shockwave therapy or a vacuum erection device (VED) improves efficacy outcomes, without any significant increase in adverse events [295]. Similarly, the association of daily tadalafil with a short-acting PDE5I (such as sildenafil) leads to improved outcomes, without any significant increase in adverse effects [296].
- Topical/Intraurethral alprostadil
The vasoactive agent alprostadil can be administered intraurethrally with two different formulations. The first delivery method is topical, using a cream that includes a permeation enhancer to facilitate absorption of alprostadil (200 and 300 μg) via the urethral meatus [297, 298]. Clinical data are still limited. Significant improvement compared to placebo was recorded for IIEF-EF domain score, SEP2 and SEP3 in a broad range of patients with mild-to-severe ED [299]. Adverse effects include penile erythema, penile burning, and pain that usually resolve within 2 hours of application. Systemic adverse effects are rare. Topical alprostadil (VITAROS™) at a dose of 300 μg is available in some European countries. Recently, a randomised cross-over clinical trial has shown that, compared to the standard administration route, direct delivery within the urethral meatus can increase efficacy and confidence among patients, without increasing adverse effects [300]. The second delivery method is by intra-urethral insertion of a specific formulation of alprostadil (125-1000 μg) in a medicated pellet (MUSE™) [44]. Erections sufficient for intercourse are achieved in 30-65.9% of patients. In clinical practice, it is recommended that intra-urethral alprostadil is initiated at a dose of 500 μg, as it has a higher efficacy than the 250 μg dose, with minimal differences with regard to adverse events. In case of unsatisfactory clinical response, the dose can be increased to 1000 μg [301–303]. The application of a constriction ring at the root of the penis may improve efficacy [302, 303]. Overall, the most common adverse events are local pain (29-41%) and dizziness with possible hypotension (1.9-14%). Penile fibrosis and priapism are rare (< 1%). Urethral bleeding (5%) and urinary tract infections (0.2%) are adverse events related to the mode of administration. Efficacy rates are significantly lower than for intracavernous pharmacotherapy [304], with ~30% adherence to long-term therapy. Intraurethral pharmacotherapy provides an alternative to intracavernous injections in patients who prefer a less-invasive, although less-efficacious treatment.
- Shockwave therapy
The use of LI-SWT has been increasingly proposed as a treatment for vasculogenic ED over the last decade, being the only currently marketed treatment that might offer a cure, which is the most desired outcome for most men suffering from ED [299, 305–312]. Overall, several single-arm trials have shown a beneficial effect of LI-SWT on patient-reported erectile function, but data from prospective randomised trials are conflicting, and many questions remain to be answered especially because of the heterogeneity among shockwave generators (i.e., electrohydraulic, electromagnetic, piezoelectric and electropneumatic); type of shockwaves delivered (i.e., focused, linear, semi-focused and unfocused); set-up parameters (e.g., energy flux density and number of pulses per session) and treatment protocols (i.e., duration of treatment, number of sessions per week, total number of shockwave pulses delivered and penile sites of application) [313, 314]. In a recent trial trying to assess the best treatment parameters, no significant differences were observed between various energy flux density levels although a 0.10 mJ/mm2 seems to perform slightly better than lower energies [315]. Most of the studies have suggested that LI-SWT can significantly increase the IIEF and EHS in patients with mild vasculogenic ED, although this improvement appears modest and the rates of patients reporting a satisfactory improvement range between 40-80% [200, 313]. Few studies have shown an improvement in penile haemodynamic parameters after LI-SWT, but the clinical meaning of this improvement remains unclear [313, 316]. Likewise, data suggest that LI-SWT could ameliorate erection quality even in patients with severe ED who are either PDE5Is nonresponders [310, 317, 318] or inadequate responders [568], thus reducing the immediate need for more invasive treatments. Treatment effect appears to be clinically evident starting from 1-3 months after treatment completion, with a subsequent progressive decrease of the achieved benefit in terms of erectile function over time, although some effects could be still detected up to 5 years after treatment [313, 315, 319]. Recently, the impact of LI-SWT has been also tested in the setting of penile rehabilitation after radical prostatectomy in 2 small, randomised trials showing only modest advantage compared to conventional PDE5is [320, 321]. Overall, larger prospective RCTs and longer-term follow-up data are necessary to provide clinicians with more confidence regarding the use and effectiveness of LI-SWT for ED. Further clarity is also needed in defining treatment protocols that can result in greater clinical benefits [322, 323]. As a whole, according to the available data and the novel treatment decision algorithm, LI-SWT may be offered to patients with vasculogenic ED, although they should be fully counselled before treatment.
- Psychosocial intervention and therapy
Psychosocial interventions including different modalities (e.g., sexual skills training, marital therapy, psychosexual education) [212], and Cognitive and Behavioural Therapy (CBT – group or couple format), are recommended [210]. Cognitive and Behaviour Therapy is aimed at altering dysfunctional cognitive and behavioural patterns influencing ED, and increasing adjustment during the course of the disorder. Some of its techniques include identifying triggers preceding erectile difficulties, cognitive restructuring of dysfunctional thinking styles, learning coping skills aimed at dealing with erectile difficulties and emotional symptoms, improving communications skills with the partner, and relapse prevention. The CBT approach combined with medical treatment for ED has received empirical support and is considered an optimal procedure [324]. Moreover, there is preliminary evidence supporting the role of mindfulness-based therapy for ED and associated outcomes such as sexual satisfaction [325].
- Hormonal treatment
The advice of an endocrinologist should be sought for managing patients with certain hormonal abnormalities or endocrinopathies [184]. Testosterone deficiency is either a result of primary testicular failure or secondary to pituitary/hypothalamic causes (e.g., a functional pituitary tumour resulting in hyperprolactinaemia) [184, 326]. When clinically indicated [327], testosterone therapy (intramuscular, transdermal, or oral) can be considered for men with low or low-normal testosterone levels and concomitant problems with their sexual desire, erectile function and dissatisfaction derived from intercourse and overall sex life.
- Vacuum erection devices
Vacuum erection devices (VED) provide passive engorgement of the corpus cavernosum, together with a constrictor ring placed at the base of the penis to retain blood within the corpus. Published data report that efficacy, in terms of erections satisfactory for intercourse, is as high as 90%, regardless of the cause of ED and satisfaction rates range between 27% and 94% [328, 329]. Most men who discontinue use of VEDs do so within 3 months. Long-term use of VEDs decreases to 50-64% after 2 years [330]. The most common adverse events include pain, inability to ejaculate, petechiae, bruising, and numbness [329]. Serious adverse events (skin necrosis) can be avoided if patients remove the constriction ring within 30 minutes. Vacuum erection devices are contraindicated in patients with bleeding disorders or on anticoagulant therapy [331, 332]. Vacuum erection devices may be the treatment of choice in well-informed older patients with infrequent sexual intercourse and co-morbidity requiring non-invasive, drug-free management of ED [328, 329, 333].
- Intracavernous injections therapy
Intracavernous administration of vasoactive drugs was the first medical treatment introduced for ED [283, 334]. According to invasiveness, tolerability, effectiveness and patients’ expectations (Figure 4), patients may be offered intracavernous injections. The success rate is high (85%) [304, 335].
- Alprostadil
Alprostadil (Caverject™, Edex/Viridal™) was the first and only drug approved for intracavernous treatment of ED [283, 336]. Intracavernous alprostadil is most efficacious as a monotherapy at a dose of 5-40 μg (40 μg may be offered off label in some European countries). The erection appears after 5-15 minutes and lasts according to the dose injected, but with significant heterogeneity among patients. An office-training programme is required for patients to learn the injection technique. In men with limited manual dexterity, the technique may be taught to their partners. The use of an automatic pen that avoids a view of the needle may be useful to resolve fear of penile puncture and simplifies the technique. Efficacy rates for intracavernous alprostadil of > 70% have been found in the general ED population, as well as in patient subgroups (e.g., men with diabetes or CVD), with reported satisfaction rates of 87-93.5% in patients and 86-90.3% in partners after the injections [283, 334]. Complications of intracavernous alprostadil include penile pain (50% of patients reported pain only after 11% of total injections), excessively-prolonged undesired erections (5%), priapism (1%), and fibrosis (2%) [283, 334, 337]. Pain is usually self-limited after prolonged use and it can be alleviated with the addition of sodium bicarbonate or local anaesthesia [283, 334, 338]. Cavernosal fibrosis (from a small haematoma) usually clears within a few months after temporary discontinuation of the injection programme. However, tunical fibrosis suggests early onset of Peyronie’s disease and may indicate stopping intracavernous injections indefinitely. Systemic adverse effects are uncommon. The most common is mild hypotension, especially when using higher doses. Contraindications include men with a history of hypersensitivity to alprostadil, men at risk of priapism, and men with bleeding disorders. Despite these favourable data, drop-out rates of 41-68% have been reported for intracavernous pharmacotherapy [283, 334, 339, 340], with most drop-outs occurring within the first 2-3 three months. In a comparative study, alprostadil monotherapy had the lowest discontinuation rate (27.5%) compared to overall drug combinations (37.6%), with an attrition rate after the first few months of therapy of 10% per year [341]. Reasons for discontinuation included desire for a permanent mode of therapy (29%), lack of a suitable partner (26%), poor response (23%) (especially among early drop-out patients), fear of needles (23%), fear of complications (22%), and lack of spontaneity (21%). Careful counselling of patients during the office-training phase as well as close follow-up are important in addressing patient withdrawal from an intracavernous injection programme [342–344].
- Combination therapy
Table 8 details the available intracavernous injection therapies (compounds and characteristics). Combination therapy enables a patient to take advantage of the different modes of action of the drugs being used, as well as alleviating adverse effects by using lower doses of each drug.
Table 8: Intracavernous injection therapy – compounds and characteristics
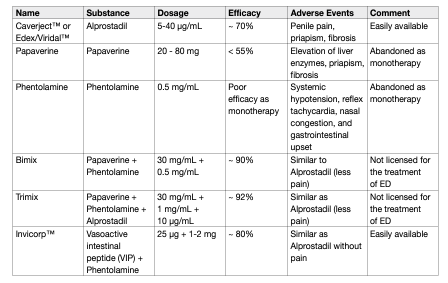
• Papaverine (20-80 mg) was the first oral drug used for intracavernous injections. It is most commonly used in combination therapy because of its high incidence of adverse effects as monotherapy. Papaverine is currently not licensed for treatment of ED.
• Phentolamine has been used in combination therapy to increase efficacy. As monotherapy, it produces a poor erectile response.
• Sparse data in the literature support the use of other drugs, such as vasoactive intestinal peptide (VIP), NO donors (linsidomine), forskolin, potassium channel openers, moxisylyte or calcitonin gene-related peptide, usually combined with the main drugs [345, 346]. Most combinations are not standardised and some drugs have limited availability worldwide.
• Bimix, Trimix: papaverine (7.5-45 mg) plus phentolamine (0.25-1.5 mg) (also known as Bimix), and papaverine (8-16 mg) plus phentolamine (0.2-0.4 mg) plus alprostadil (10-20 μg) (also known as Trimix), have been widely used with improved efficacy rates, although they have never been licensed for ED [347, 348]. Trimix has the highest efficacy rates, reaching 92%; this combination has similar adverse effects as alprostadil monotherapy, but a lower incidence of penile pain due to lower doses of alprostadil. However, fibrosis is more common (5-10%) when papaverine is used (depending on total dose).
• Invicorp™: Vasoactive intestinal peptide (25 μg) plus phentolamine mesylate (1-2 mg Invicorp), currently licensed in Scandinavia, is a combination of two active components with complementary modes of action. Clinical studies have shown that the combination is effective for intracavernous injections in > 80% of men with ED, including those who have failed to respond to other therapies and, unlike existing intracavernous therapies, is associated with a low incidence of penile pain and a virtually negligible risk of priapism [349]. Despite high efficacy rates, 5-10% of patients do not respond to combination intracavernous injections. The combination of sildenafil with intracavernous injection of the triple combination regimen may salvage as many as 31% of patients who do not respond to the triple combination alone [350]. However, combination therapy is associated with an increased incidence of adverse effects in 33% of patients, including dizziness in 20% of patients. This strategy can be considered in carefully selected patients before proceeding to a penile implant.
Other treatments:
- Platelet-Rich Plasma
The interest toward regenerative medicine for ED has significantly increased in the last decade [351]. Among these, intracavernous injection of platelet-rich plasma (PRP) has been recently investigated in several prospective and retrospective trials [352–359]. Platelet-rich plasma is obtained by centrifugation of patient autologous blood with subsequent extraction of a plasma fraction containing 3-7 times mean platelet concentration compared to the whole blood. The regenerative effect of PRP is deemed to be exerted through the high concentrations of platelets containing several growth factors including VEGF, EGF, IGF-1, PDGF and FGF [360]. These factors may be responsible for angiogenesis stimulation and stem cell recruitment [360]. Pre-clinical studies have shown a neuro-regenerative effect and an improved penile vascularisation in both cavernous nerve injury and diabetic rat-model [361]. In the clinical setting, the use of PRP has been previously investigated in the field of orthopaedics, plastic surgery and dermatology. To date, one randomised placebo controlled-trial [359], two prospective randomised trials [355, 356], two prospective cohort [352, 358] and two retrospective studies [34, 357] investigated the effect of intracavernous injection of PRP for ED. Overall, available findings demonstrate favourable outcomes of PRP injections in terms of IIEF-5 and SEP scores and peak systolic velocity on penile-duplex ultrasound [361]. In the only randomised placebocontrolled trial, 60 patients with mild to moderate vasculogenic ED were randomised to receive two injections of 10 mL PRP (n=30) or placebo (n=30) [359]. At 1, 3 and 6-month follow-up, the rate of patients reporting an MCID improvement in the IIEF-EF score was significantly higher in the treatment group, with 69% achieving minimal clinically important differences (MCID) 6 months after PRP compared to 27% in the placebo group (p < 0.001). IIEF-EF scores improved by a mean of 2.7 points at 1-month and 3.9 points at 6-month assessment after treatment. Regarding safety, the mean VAS score was higher as compared with placebo (2.6 vs. 2.2, respectively, p = 0.008) but no haemorrhagic events or other side effects were reported [359]. Despite these encouraging results, the available evidence is still insufficient to provide a recommendation regarding the use of PRP for ED treatment in clinical practice. Indeed, current studies are limited by the low number of patients included (ranging from 10-100), the lack of placebo comparison (except for 1 small RCT) and the heterogeneity in terms of the modality of PRP preparation. The concentration of platelets and growth factors could vary according to the system used for preparation [362] and there is a lack of consensus concerning the optimal platelet concentration as well as the need for combining PRP with activating agents such as CaCl2 or thrombin to maximise the growth factors release [361, 362]. Intracavernous injection of PRP should be used only in a clinical trial setting.
- Herbal medicine and natural supplements
In recent years there has been an exponential growth in the market of medicinal herbs and natural supplements for the treatment of ED, but with very little available evidence of robust scientific data to support their efficacy and safety. Recently, a Cochrane review showed that ginseng may only have trivial effects on erectile function or satisfaction with intercourse compared to placebo when assessed using validated tools [363]. Moreover, data suggested that daily administration of oral L-arginine, only when in combination with PDE5I use, improves sexual function [364].
- Erectile dysfunction after radical prostatectomy
Use of pro-erectile drugs following RP is important in achieving post-operative erectile function and to allow patients to resume sexual activity. There is also some evidence in animal studies that this may avoid cavernous fibrosis and maintain penile length. Several trials have shown improvements in erectile function after RP in patients receiving drugs (any therapeutic or prophylactic) for ED. Early compared with delayed erectile function treatment affects the natural recovery time for potency [365], although there is a lack of data to support any specific regimen, which is either optimal for penile rehabilitation or may result in the achievement of spontaneous, non-pharmacologically assisted erections [144, 366, 367]. In prospective studies, there has been no evidence that penile rehabilitation itself increases the chances of spontaneous recovery of erectile function in men following nerve-sparing RP (NSRP) [367]. The currently available therapeutic armamentarium follows the treatment algorithm for ED, which is shown in Figure 2. Management of post-RP ED has been revolutionised by the advent of PDE5Is, with their demonstrated efficacy, ease of use, good tolerability, excellent safety, and positive impact on QoL. In this context, it must be emphasised that post-RP, ED patients are poor responders to PDE5Is. Since their launch on the market, PDE5Is have been considered as the first-line therapy in patients who have undergone NS surgery, regardless of the surgical technique used [144, 151]. Several clinical parameters have been identified as potential predictors of PDE5Is outcomes in men undergoing RP. Patient age, baseline erectile function, and quality of NS technique are key factors in preserving post-RP erectile function [151, 160, 368]. The response rate to sildenafil treatment for ED after RP in different trials has ranged from 35-75% among those who underwent NSRP and from 0-15% among those who underwent non-NSRP [151, 369]. Early use of high-dose sildenafil after RP is associated with preservation of smooth muscle within the corpus cavernosum [370]. A single study demonstrated that daily sildenafil also results in a greater return of spontaneous normal erectile function after RP compared to placebo following bilateral NSRP in patients who were fully potent before surgery [371]. Conversely, a more recent prospective, randomised, placebo-controlled study, which assessed the effects of nightly sildenafil citrate therapy during penile rehabilitation using nocturnal penile rigidity score in addition to the IIEF-EF domain showed no therapeutic benefit for nightly sildenafil when compared to on-demand dosing in recovery of erectile function post-prostatectomy [372]. A large multicentre trial in Europe and the USA investigated the effects of tadalafil in patients with ED following bilateral NSRP. Erectile function was improved in 71% of patients treated with 20 mg tadalafil versus 24% of those treated with placebo, while the rate of successful intercourse attempts was 52% with 20 mg tadalafil vs. 26% with placebo [373]. Moreover, a randomised, double-blind, double-placebo trial in men < 68 years of age and with normal pre-operative erectile function who underwent NSRP at 50 centres from nine European countries and Canada, compared tadalafil once daily with placebo [367]. Tadalafil was most effective for drugassisted erectile function in men with ED following NSRP and data suggested a potential role for tadalafil once daily (provided early after surgery) in contributing to the recovery of post-operative erectile function and maintaining penile length [367]. Conversely, unassisted or spontaneous recovery of erectile function was not improved after cessation of active therapy for 9 months [367]. However, tadalafil once daily improved QoL post-operatively, both at double-blind and open label treatment periods [374]. Similarly, vardenafil has been tested in patients with ED following NSRP in a randomised, multicentre, prospective, placebo-controlled study [375]. Following bilateral NSRP, erectile function improved by 71% and 60% with 10 and 20 mg vardenafil, respectively. An extended analysis of the same cohort of patients showed the benefit of vardenafil compared to placebo in terms of intercourse satisfaction, hardness of erection, orgasmic function, and overall satisfaction with sexual experience [376]. A randomised, double-blind, double-dummy, multicentre, parallel-group study in 87 centres across Europe, Canada, South Africa and the USA, compared on-demand and nightly dosing of vardenafil in men with ED following bilateral NSRP [366]. In patients whose preoperative erectile function domain score was > 26, vardenafil was efficacious when used on demand [366]. A double-blind, placebo-controlled, parallel-group study in 298 patients with ED after bilateral NSRP randomised to 100 or 200 mg avanafil or placebo (30 minutes before sexual activity) for 12 weeks showed significantly greater increases in SEP2 and SEP3 as well as in mean change of IIEF erectile function domain score with 100 and 200 mg avanafil versus placebo (P < 0.01) [171]. A recent Cochrane review analysed data from eight RCTs [377]. It showed that scheduled PDE5I may have little or no effect on short-term (up to 12 months) self-reported potency when compared to placebo or no treatment. In this study, daily PDE5I made little to no difference in short- and long-term erectile function. The authors conclude that penile rehabilitation strategies using PDE5I following RP do not increase self-reported potency and erectile function compared to on-demand use. Therefore, daily PDE5Is result in little to no difference in both short- and long-term (> 12 months) self-reported potency when compared to scheduled use. Finally, at short-term follow-up, daily PDE5I may result in little or no effect on self-reported potency when compared to scheduled intra-urethral application of prostaglandin E1. Historically, the treatment options for post-RP ED have included intracavernous injections [378], urethral micro-suppository [151, 379], VED [144, 151, 380, 381], and penile implants [151, 382, 383]. Intracavernous injections and penile implants had been suggested as second- and third-line treatments, respectively, when oral PDE5Is are not adequately effective or not suitable for post-operative patients [144, 384]. A meta-analysis had shown that the early use of VED has an excellent therapeutic effect on post-RP patients and no serious adverse effects, therefore it should be considered as a therapeutic alternative [385]. Recent findings from two network meta-analyses show that: i) Sildenafil 100 mg regular dose (once daily or nightly) is the optimum penile rehabilitation strategy to improve erectile function recovery rates after RP, while the on-demand dose of PDE5Is should not be considered and recommended as a penile rehabilitation strategy [386]; ii) the combination therapy with VED and PDE5is offers clear advantages over monotherapy, thus this combined approach should be considered in the clinical management of penile rehabilitation after RP [387]. Findings from a systematic review had suggested that pelvic floor muscle training (PFMT) combined with biofeedback is a promising alternative to pharmacological treatments, although there is a need for future wellpowered, rigorously designed RCTs to draw strong conclusions [388].
Surgical management:
- Surgery for post-traumatic arteriogenic ED
In young patients with pelvic or perineal trauma, surgical penile revascularisation has a 60-70% long-term success rate [332, 389]. The stenosis must be confirmed by penile pharmaco-arteriography. Corporeal venoocclusive dysfunction is a contraindication to revascularisation and must be excluded by dynamic infusion cavernosometry or cavernosography.
- Venous ligation surgery
Venous ligation surgery for veno-occlusive dysfunction is no longer recommended because of poor long-term results [389].
- Penile prostheses
The surgical implantation of a penile prosthesis may be considered in patients who i) are not suitable for different pharmacotherapies or prefer a definitive therapy; and, ii) do not respond to pharmacological therapies (Figure 4) [390]. A systematic review addressing cause and duration of symptoms before implantation has shown that most men receiving a penile prosthesis have an organic cause of ED, with vascular disease, diabetes, and previous pelvic surgery/trauma being the most common [391]. Similar findings have been reported by a prospective registry of penile prostheses with > 3-year collection period in the UK; the three commonest aetiological factors for ED were diabetes, prostate surgery and Peyronie’s disease [392]. The mean duration of ED symptoms before surgical intervention ranges from 3-6 six years [391]. The two currently available classes of penile implants include inflatable (two- and three-piece) and semi-rigid devices (malleable, mechanical and soft flexible) [151, 382, 393–395]. Patients may prefer the three-piece inflatable devices due to the more “natural” erections obtained, although no prospective RCTs have compared satisfaction rates with both types of implants. The two-piece inflatable prosthesis can be a viable option among patients who are deemed at high-risk of complications with reservoir placements (e.g., previous abdominal surgery). Semi-rigid prostheses result in a firm penis, which may be manually placed in an erect or flaccid state and offer the advantage of a simple implant technique, as well as easy use for the patient [151, 382, 393, 394]. Conversely, they can have the disadvantage of unnatural persistent erection and reduced concealability [394, 396]. They may also be an option in men with limited manual dexterity. There are two main surgical approaches for penile prosthesis implantation: peno-scrotal and infrapubic [393, 394, 396, 397]. The peno-scrotal approach has been suggested to provide an excellent exposure; afford proximal crural exposure, avoid dorsal nerve injury, and permit direct visualisation of pump placement. However, with this approach, the reservoir is either placed blindly into the retropubic space, which can result in visceral injury in patients with a history of major pelvic surgery (mainly radical cystectomy) or a separate incision in the abdomen is placed under direct vision. A recent systematic review comparing the satisfaction and complication rates of the different surgical approaches has shown that there is no specific advantage between the two, but rather it is recommended that surgeons have knowledge of both techniques and are capable of tailoring the incision strategy for complex cases [398]. Revision surgery is associated with poorer outcomes and may be more challenging. Regardless of the indication, prosthesis implantation has one of the highest satisfaction rates (92-100% in patients and 91-95% in partners) among the treatment options for ED with appropriate counselling [151, 382, 393, 399–406]. In patients with favourable oncological prognosis after RP for PCa, a contemporary surgery to treat both ED, with the implant of a penile prosthesis, and stress urinary incontinence (male sling or artificial urinary sphincter) is effective and durable and has an established and definitive role to address both problems [151, 382, 407–409]. Structured psychosexual counselling may improve sexuality and sexual well-being in both patients and their partners after penile implant surgery [410].
- Penile prostheses implantation: complications
Historically, the two main complications of penile prosthesis implantation are mechanical failure and infection. Several technical modifications of the most commonly used three-piece prostheses (e.g., AMS 700CX/CXR™ and Titan Zero degree™) resulted in mechanical failure rates of < 5% after 5 years of follow-up [382, 411, 412]. Careful surgical techniques with appropriate antibiotic prophylaxis against Gram-positive and Gram-negative bacteria reduced infection rates to 2-3% with primary implantation in low-risk patients and in high-volume centres, although the definition of a high-volume centre still needs clarification [413–416]. The infection rate may be further reduced to 1-2% by implanting an antibiotic-impregnated prosthesis (AMS Inhibizone™) or hydrophilic-coated prosthesis (Coloplast Titan™) [382, 413, 417–420]. Methods that decrease infections include using coated prostheses and strictly adhering to surgical techniques that avoid prolonged wound exposure and skin contact minimisation (i.e., no-touch technique). Techniques that might prevent penile prostheses infection but lack definitive evidence include the use of prolonged post-operative antibiotics (> 24 hours), shaving with clippers, and preparation with chlorhexidinealcohol [421, 422]. Identification and pre-treatment of patients who are colonised with nasal Staphylococcus aureus with mupirocin and chlorhexidine prior to surgery has been shown to reduce the incidence of postoperative surgical site infection from 4.4% to 0.9% in a placebo-controlled randomised trial [423]. On the whole, growing evidence suggests that the risk of penile prosthesis infection has reduced over the last few decades with device improvement and surgical expertise [424]. Higher-risk populations include patients undergoing revision surgery, those with impaired host defences (immunosuppression, diabetes mellitus, or spinal cord injury) or those with penile corporal fibrosis [382, 393, 414, 425–427]. A recent large database-study has shown that diabetes mellitus is a risk factor for penile prostheses infection, highlighting the need for optimal patient selection other than raising the question of whether lowering this risk by optimising glycaemic control before surgery [428]. Unfortunately, there are no RCTs determining the ideal and/or correct threshold of glycated haemoglobin that is acceptable prior to implant surgery in diabetic patients [429]. Recently, a large-cohort, multicentre, retrospective analysis in men with diabetes who received a Coloplast Titan™ implant demonstrated that vancomycin + gentamicin was the most efficacious combination of antibiotics used for implants dipping in terms of preventing postoperative infection and subsequent explantation and revision [430, 431]. Infection requires removal of the prosthesis and antibiotic administration. Alternatively, removal of the infected device with immediate salvage and replacement with a new prosthesis has been described using a wash-out protocol with successful salvages achieved in > 80% of cases [414, 426, 432, 433]. An absolute recommendation on how to proceed after explantation in this setting cannot be given and must be focused on the pros and cons of salvage therapy after full consultation with the patient. The majority of revisions are secondary to mechanical failure and combined erosion or infection [419, 421]. Ninety-three percent of cases are successfully revised, providing functioning penile prosthesis [414, 419, 432, 434, 435].
Besides infection and mechanical failure, impending erosion involving the distal corpora, urethra, glans or other structures can occur in 1-6% of cases after surgery [436]. Similarly, glans ischaemia and necrosis have been reported in about 1.5% of patients [436, 437]. Risk factors for these serious complications are higher in those patients with significant vascular impairment, such as patients with diabetes, or who have undergone concomitant lengthening procedures. Therefore, performing dual procedures at the time of implantation should be limited to mitigate the risks of serious complications.
- Conclusions about penile prostheses implantation
Penile implants are an effective solution, usually for patients who do not respond to more conservative therapies. There is sufficient evidence to recommend this approach in patients not responding to less-invasive treatments due to its high efficacy, safety and satisfaction rate [438]. There are also currently no head-to-head studies comparing the different manufacturers’ implants, demonstrating superiority of one implant type over another [439].
Table 9: Penile prostheses models available on the market
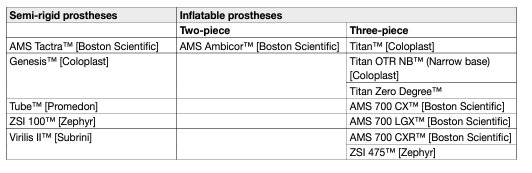
- Follow-up
Follow-up is important in order to assess efficacy and safety of the treatment provided. It is also essential to assess patient satisfaction since successful treatment for ED goes beyond efficacy and safety. Physicians must be aware that there is no single treatment that fits all patients or all situations.
DISORDERS OF EJACULATION:
- Introduction
Ejaculation is a complex physiological process that comprises emission and expulsion processes and is mediated by interwoven neurological and hormonal pathways [440]. Any interference with those pathways may cause a wide range of ejaculatory disorders (Table 10).
Table 10: Spectrum of ejaculation disorders

- Pathophysiology and risk factors
The aetiology of PE is unknown, with few data to support suggested biological and psychological hypotheses, including anxiety [441–445], penile hypersensitivity [446–450] and 5-hydroxytryptamine (HT) receptor dysfunction [453–458]. The classification of PE into four subtypes [16] has contributed to a better delineation of lifelong, acquired, variable and subjective PE [459–461]. It has been hypothesised that the pathophysiology of lifelong PE is mediated by a complex interplay of central and peripheral serotonergic, dopaminergic, oxytocinergic, endocrinological, genetic and epigenetic factors [462]. Acquired PE may occur due to psychological problems – such as sexual performance anxiety, and psychological or relationship problems – and/or co-morbidity, including ED, prostatitis and hyperthyroidism [463–465]. A significant proportion of men with ED also experience PE [24, 128]. High levels of performance anxiety related to ED may worsen PE, with a risk of misdiagnosing PE instead of the underlying ED. According to the National Health and Social Life Survey (NHSLS), the prevalence of PE is not affected by age [12], unlike ED, which increases with age. Conversely, other data depicted an increased prevalence with ageing [445]; for instance, Verze et al. reported that PE prevalence based on the Premature Ejaculation Diagnostic Tool (PEDT) score (> 11) [466] proportionally increased with age [467]. Similarly, in a recent systematic review, PE was found to be more common in older age, with peak prevalence in men aged 60-69 years [468]. Premature ejaculation is not affected by marital or income status [12, 467]. However, PE is more common in Black men, Hispanic men, and men from regions where an Islamic background is common [11, 469] and prevalence may be higher in men with a lower educational level [12, 24]. Other risk factors include genetic predisposition [458, 470–473], poor overall health status and obesity [12], prostate inflammation [107, 474–477], hyperthyroidism [463], low prolactin levels [478], high testosterone levels [479], vitamin D and B12 deficiency [480, 481], diabetes [482, 483], MetS [484, 485], lack of physical activity [486], emotional problems and stress [12, 487, 488], depressive symptoms [488], and traumatic sexual experiences [12, 24]. In the only published study on risk modification/ prevention strategies [489], successful eradication of causative organisms in patients with chronic prostatitis and PE produced marked improvements in intravaginal ejaculatory latency time (IELT) and ejaculatory control compared to untreated patients.
- Impact of PE on quality-of-life
Men with PE are more likely to report low satisfaction with their sexual relationship, low satisfaction with sexual intercourse, difficulty relaxing during intercourse, and less-frequent intercourse [490, 491, 492]. However, the negative impact of PE extends beyond sexual dysfunction. Premature ejaculation can have a detrimental effect on self-confidence and the relationship with the partner, and may sometimes cause mental distress, anxiety, embarrassment and depression [490, 493, 494]. Moreover, PE may also affect the partner’s sexual functioning and their satisfaction with the sexual relationship decreases with increasing severity of the patient’s condition [495–497]. Despite the possible serious psychological and QoL consequences of PE, few men seek treatment. In the Global Study of Sexual Attitudes and Behaviors survey, 78% of men who self-reported a sexual dysfunction sought no professional help or advice for their sexual problems [24], with men more likely to seek treatment for ED than for PE [24]. In the Premature Ejaculation Prevalence and Attitudes (PEPA) survey, only 9% of men with self-reported PE consulted a physician [13]. The main reasons for not discussing PE with their physician are embarrassment and a belief that there is no treatment. Physicians are often uncomfortable discussing sexuality with their patients usually because of embarrassment and a lack of training or expertise in treating PE [498, 499]. Physicians need to encourage their patients to talk about PE.
- Classification
There is still little consensus about the definition and classification of PE [500]. It is now universally accepted that “premature ejaculation” is a broad term that includes several concepts belonging to the common category of PE. The most recent definition comes from the International Classification of Diseases 11th Revision, where PE was renamed as Early Ejaculation [501]: ‘’Male early ejaculation is characterized by ejaculation that occurs prior to or within a very short duration of the initiation of vaginal penetration or other relevant sexual stimulation, with no or little perceived control over ejaculation. The pattern of early ejaculation has occurred episodically or persistently over a period of at least several months and is associated with clinically significant distress.”
This definition includes four categories: male early ejaculation, lifelong generalised and situational, acquired generalised and situational, unspecified. In the Diagnostic and Statistical Manual of Mental Disorders V (DSM-V), PE is defined as a sexual disorder with:
• consistent ejaculation within 1 minute or less of vaginal penetration;
• over a period of at least 6 months;
• experienced 75–100% of the time;
• the condition results in clinically significant distress, sexual frustration, dissatisfaction, or tension between partners;
• this condition is not better accounted for by another non-sexual mental disorder, medication or illicit substance use, or medical condition [31].
The EAU Guidelines have adopted the definition of PE that was developed by the International Society for Sexual Medicine as the first evidence-based definition [502]. According to this definition, PE (lifelong and acquired) is a male sexual dysfunction characterised by the following:
• ejaculation that always or nearly always occurs prior to or within about 1 minute of vaginal penetration (lifelong PE) or a clinically significant and bothersome reduction in latency time, often to about 3 minutes or less (acquired PE);
• inability to delay ejaculation on all or nearly all vaginal penetrations;
• negative personal consequences, such as distress, bother, frustration, and/or the avoidance of sexual intimacy.
Two more PE syndromes have been proposed [460]:
• ‘Variable PE’ is characterised by inconsistent and irregular early ejaculations, representing a normal variation in sexual performance.
• ‘Subjective PE’ is characterised by subjective perception of consistent or inconsistent rapid ejaculation during intercourse, while ejaculation latency time is in the normal range or can even last longer. It should not be regarded as a symptom or manifestation of true medical pathology.
The addition of these new syndrome types may help in overcoming the limitations of each individual definition and it may support a more flexible view of PE for patient stratification, diagnosis and treatment [503].
- Diagnostic evaluation
Diagnosis of PE is based on the patient’s medical and sexual history [20, 504, 505]. History should classify PE as lifelong or acquired and determine whether PE is situational (under specific circumstances or with a specific partner) or consistent. Special attention should be given to the duration time of ejaculation, degree of sexual stimulus, impact on sexual activity and QoL, and drug use or abuse. It is also important to distinguish PE from ED. Many patients with ED develop secondary PE caused by the anxiety associated with difficulty in attaining and maintaining an erection [128, 506]. Furthermore, some patients are not aware that loss of erection after ejaculation is normal and may erroneously complain of ED, while the actual problem is PE [507]. There are several overlapping definitions of PE, with four shared factors (Table 11), resulting in a multi-dimensional diagnosis [508].
Table 11: Common factors in different definitions of PE
- Intravaginal ejaculatory latency time (IELT)
Although it has been suggested as an objective diagnostic criterion and treatment outcome measure [509, 510], the use of IELT alone is not sufficient to define PE, as there is significant overlap between men with and without PE [511, 512]. Moreover, some men may experience PE in their non-coital sexual activities (e.g., during masturbation, oral sex or anal intercourse) thus measuring IELT will not be suitable for their assessment. Intravaginal ejaculatory latency time has a significant direct effect on perceived control over ejaculation, but not a significant direct effect on ejaculation-related personal distress or satisfaction with sexual intercourse [491]. In addition, perceived control over ejaculation has a significant direct effect on both ejaculation-related personal distress and satisfaction with sexual intercourse (each showing direct effects on interpersonal difficulty related to ejaculation) [513].
In everyday clinical practice, self-estimated IELT is sufficient [8]. Self-estimated and stopwatch-measured IELT are interchangeable and correctly assign PE status with 80% sensitivity and 80% specificity [514]. Specificity can be improved further to 96% by combining IELT with a single-item patientreported outcome (PRO) scale on control over ejaculation and satisfaction with sexual intercourse (0 = very poor, to 4 = very good) and on personal distress and interpersonal difficulty (0 = not at all, to 4 = extremely). However, self-estimated IELT may be over-estimated by ~1 minute and therefore it must be carefully substituted with stopwatch-measured IELT while identifying men with the complaint of lifelong PE in a clinical setting [515].
- Physical examination and investigations
Physical examination may be part of the initial assessment of men with PE. It may include a focused examination of the urological, endocrine and neurological systems to identify underlying medical conditions associated with PE or other sexual dysfunctions, such as endocrinopathy, Peyronie’s disease, urethritis or prostatitis. Laboratory or physiological testing should be directed by specific findings from history or physical examination and is not routinely recommended [504].
- Disease management
Before commencing any treatment, it is essential to define the subtype of PE and discuss patient’s expectations thoroughly. Pharmacotherapy must be considered as the first-line treatment for patients with lifelong PE, whereas treating the underlying cause (e.g., ED, prostatitis, LUTS, anxiety and hyperthyroidism) must be the initial goal for patients with acquired PE [20]. Various behavioural techniques may be beneficial in treating variable and subjective PE [516]. Psychotherapy can also be considered for PE patients who are uncomfortable with pharmacological therapy or in combination with pharmacological therapy [517, 518]. However, there is weak and inconsistent evidence regarding the effectiveness of these psychosexual interventions and their long-term outcomes in PE are unknown [519]. In lifelong PE, behavioural techniques are not recommended alone, and pharmacotherapy must be considered as the basis of treatment [20]. Dapoxetine (30 and 60 mg) is the first on-demand oral pharmacological agent approved for lifelong and acquired PE in many countries, except for the USA [520]. The metered-dose aerosol spray of lidocaine (150 mg/mL) and prilocaine (50 mg/mL) combination is the first topical formulation to be officially approved for on-demand treatment of lifelong PE by the EMA in the European Union [521]. All other medications used in PE are off-label indications [522]. Daily or on-demand use of selective serotonin re-uptake inhibitors (SSRIs) and clomipramine, and on-demand topical anaesthetic agents have consistently shown efficacy in PE [523–526]. Long-term outcomes of pharmacological treatments are unknown. An evidence-based analysis of all current treatment modalities was performed. Levels of evidence and grades of recommendation are provided, and a treatment algorithm is presented (Figure 5).
Figure 5: Management of premature ejaculation
Pharmacotherapy:
- Dapoxetine
Dapoxetine hydrochloride is a short-acting SSRI, with a pharmacokinetic profile suitable for on-demand treatment for PE [522]. It has a rapid Tmax (1.3 hours) and a short half-life (95% clearance rate after 24 hours) [527, 528]. It is approved for on-demand treatment of PE in European countries and elsewhere, but not in the USA. Both available doses of dapoxetine (30 mg and 60 mg) have shown 2.5- and 3.0-fold increases, respectively, in IELT overall, rising to 3.4- and 4.3-fold in patients with a baseline average IELT < 30 seconds [520, 529, 530]. In RCTs, dapoxetine, 30 mg or 60 mg 1-2 hours before intercourse, was effective at improving IELT and increasing ejaculatory control, decreasing distress, and increasing satisfaction [529].
- Topical anaesthetic agents
The use of local anaesthetics to delay ejaculation is the oldest form of pharmacological therapy for PE [531]. Several trials [449, 532, 533] support the hypothesis that topical desensitising agents reduce the sensitivity of the glans penis thereby delaying ejaculatory latency, but without adversely affecting the sensation of ejaculation. Meta-analyses have confirmed the efficacy and safety of these agents for the treatment of PE [534, 535]. In a recent meta-analysis, the efficacy of local anaesthetics was best among the other treatment options including SSRIs, dapoxetine 30 and 60 mg, PDE5Is and tramadol for < 8 weeks of therapy [536].
- Lidocaine/prilocaine cream
In a randomised, double-blind, placebo-controlled trial, lidocaine/prilocaine cream increased IELT from one minute in the placebo group to 6.7 minutes in the treatment group [537].
- Lidocaine/prilocaine spray
The eutectic lidocaine/prilocaine spray is a metered-dose aerosol spray containing purely base forms of lidocaine (150 mg/mL) and prilocaine (50 mg/mL), which has been officially approved by the EMA for the treatment of lifelong PE [538].
- Phosphodiesterase type 5 inhibitors
One well-designed, randomised, double-blind, placebo-controlled study compared sildenafil to placebo in men with PE [539]. Although IELT was not significantly improved, sildenafil increased confidence, the perception of ejaculatory control and overall sexual satisfaction, reduced anxiety and the refractory time to achieve a second erection after ejaculation. Another RCT demonstrated that once-daily 5 mg tadalafil for 6 weeks was effective in improving PROs and was well tolerated by patients with PE [540]. Several open-label studies have shown that combination of PDE5Is and SSRIs is superior to SSRI monotherapy, which has also been recently confirmed by a Bayesian network meta-analysis [536]:
• Sildenafil combined with paroxetine improved IELT significantly and satisfaction vs. paroxetine alone [542];
• Sildenafil combined with sertraline improved IELT and satisfaction significantly vs. sertraline alone [543];
• Sildenafil combined with paroxetine and psychological and behavioural counselling significantly improved IELT and satisfaction in patients in whom other treatments failed [544];
• Sildenafil combined with dapoxetine (30 mg) improved IELT, satisfaction scores and PEDT vs. dapoxetine, paroxetine or sildenafil monotherapy [545];
• Tadalafil combined with paroxetine significantly improved IELT and satisfaction vs. paroxetine and tadalafil alone [546];
• Sildenafil combined with behavioural therapy significantly improved IELT and satisfaction vs. behavioural therapy alone [547].
- Other drugs
In addition to the aforementioned drugs, there is continuous research into other treatment options. Considering the abundant α1a-adrenergic receptors in seminal vesicles and the prostate, and the role of the sympathetic system in ejaculation physiology, the efficacy of selective α-blockers in the treatment of PE has been assessed [548–550]. A recent study demonstrated that wake-promoting agent modafinil may be effective in delaying ejaculation and improving PROMs [551]. The efficacy of acupuncture was compared to dapoxetine for the treatment of PE and although acupuncture showed a significant ejaculation-delaying effect, this was less effective as compared with that of dapoxetine [552].
Decreasing penile sensitivity with glans penis augmentation using hyaluronic acid for the treatment of PE was initially proposed by Korean researchers in 2004 [553], and since then has gained popularity mainly in Asian countries [554, 555]. In a randomised controlled cross-over study, hyaluronic acid glans injections were safe with a modest but significant increase in IELT [556]. However, these procedures may result in serious complications and more safety studies must be conducted before recommending this treatment to PE patients [557]. Considering the importance of central oxytocin receptors in ejaculation reflex, several researchers have assessed the efficacy and safety of oxytocin receptor antagonists in the treatment of PE [558]. Epelsiban [559] and cligosiban [560–562] have been found to be safe and mildly effective in delaying ejaculation, but further controlled trials are needed [562]. Retarded ejaculation was associated with the use of pregabalin, a new generation of gapapentinoid, as a sideeffect. In a double-blind, placebo-controlled randomised trial where the efficacy and tolerability of on-demand oral pregabalin (150 mg and 75 mg) in treatment of PE was trialled, it was found that IELTs of patients who received 150 mg pregabalin improved significantly (2.45 ± 1.43-fold) compared to those who received 75 mg pregabalin and placebo. Treatment-emergent side effects (blurred vision, dizziness, vomiting) were minimal and did not lead to drug discontinuation. The role of other proposed treatment modalities for the treatment of PE such as penis-root masturbation [563], vibrator-assisted start-stop exercises [564], transcutaneous functional electric stimulation [565], transcutaneous posterior tibial nerve stimulation [566], and practicing yoga [567] need more evidence to be considered in the clinical setting.
Delayed Ejaculation:
- Definition and classification
The American Psychiatric Association defines DE as requiring one of two symptoms as follows: marked delay, infrequency, or absence of ejaculation on 75-100% of occasions, that persists for at least 6 months, and which causes personal distress [31]. However, in a recent study, while ejaculatory latency and control were significant criteria to differentiate men with DE from those without ejaculatory disorders, bother/distress did not emerge as a significant factor [568]. Similar to PE, there are distinctions among lifelong, acquired and situational DE [31]. Although the evidence is limited, the prevalence of lifelong DE and acquired DE is estimated around 1% and 4%, respectively [32].
- Pathophysiology and risk factors
The aetiology of DE can be psychological, organic (e.g., incomplete spinal cord lesion or iatrogenic penile nerve damage), or pharmacological (e.g., SSRIs, antihypertensive drugs, or antipsychotics) [569, 570] (Table 12). Other factors that may play a role in the aetiology of DE include tactile sensitivity and tissue atrophy [571].
Although low testosterone level has been considered a risk factor in the past [572, 479], more contemporary studies have not confirmed any association between ejaculation times and serum testosterone levels [573, 574]. Idiosyncratic masturbation and lack of desire for stimuli are also proposed risk factors for DE [26-28].
Table 12: Aetiological causes of delayed ejaculation and anejaculation [575]
Investigation and treatment:
Patients should have a full medical and sexual history performed along with a detailed physical examination when evaluating for DE. It is not uncommon for clinicians to feel uncomfortable with the level of sexual information that is warranted in obtaining a full sexual history. Understanding the details of the ejaculatory response, sensation, frequency, and sexual activity/techniques; cultural context and history of the disorder; quality of the sexual response cycle (desire, arousal, ejaculation, orgasm, and refractory period); partner’s assessment of the disorder and if the partner suffers from any sexual dysfunction her/himself; and the overall satisfaction of the sexual relationship are all important to garner during history-taking [576]. Investigation by a sex therapist is often required to help obtain a complete psychological evaluation. It is incumbent on the clinician to diagnose medical pathologies that cause or contribute to DE, such as assessing the hormonal milieu, anatomy, and overall medical condition. Good communication between the sex therapist and medical practitioner is vital to successful diagnosis and treatment of DE.
- Pharmacotherapy
Several pharmacological agents, including cabergoline, bupropion, alpha-1-adrenergic agonists (pseudoephedrine, midodrine, imipramine and ephedrine), buspirone, oxytocin, testosterone, bethanechol, yohimbine, amantadine, cyproheptadine and apomorphine have been used to treat DE with varied success [577]. Unfortunately, there is no FDA or EMA approved medications to treat DE, as most of the cited research is based on case-cohort studies that were not randomised, blinded, or placebo-controlled. Many drugs have been used as both primary treatments and/or as antidotes to other medications that can cause DE. A recent survey of sexual health providers demonstrated an overall treatment success of 40% with most providers commonly using cabergoline, bupropion or oxytocin [578]. However, this survey measured the anecdotal results of practitioners and there was no proven efficacy or superiority of any drug due to a lack of placebocontrolled, randomised, blinded, comparative trials [908]. In addition to pharmacotherapy, penile vibratory stimulation (PVS) is also used as an adjunct therapy for DE [579]. Another study that used combined therapy of midodrine and PVS to increase autonomic stimulation in 158 men with spinal cord injury led to ejaculation in almost 65% of the patients [580].
Anejaculation:
- Definition and classification
Anejaculation involves the complete absence of antegrade or retrograde ejaculation. It is caused by failure of semen emission from the seminal vesicles, prostate, and ejaculatory ducts into the urethra [581]. True anejaculation is usually associated with a normal orgasmic sensation and is always associated with central or peripheral nervous system dysfunction or with drugs [582].
- Pathophysiology and risk factors
Generally, anejaculation shares similar aetiological factors with DE and retrograde ejaculation (Table 12).
- Investigation and treatment
Drug treatment for anejaculation caused by lymphadenectomy and neuropathy, or psychosexual therapy for anorgasmia, is not effective. In all these cases, and in men who have a spinal cord injury, PVS (i.e., application of a vibrator to the penis) is the first-line therapy. In anejaculation, PVS evokes the ejaculation reflex [583], which requires an intact lumbosacral spinal cord segment. If the quality of semen is poor, or ejaculation is retrograde, the couple may enter an in vitro fertilisation program whenever fathering is desired. If PVS has failed, electro-ejaculation can be the therapy of choice [584]. When electro-ejaculation fails or cannot be carried out, other sperm-retrieval techniques may be used [585]. Anejaculation following either retroperitoneal surgery for testicular cancer or total mesorectal excision can be prevented using unilateral lymphadenectomy or autonomic nerve preservation [586], respectively.
Painful Ejaculation:
- Definition and classification
Painful ejaculation is a condition in which a patient feels mild discomfort to severe pain during or after ejaculation. The pain can involve the penis, scrotum, and perineum [587].
- Pathophysiology and risk factors
Many medical conditions can result in painful ejaculations, but it can also be an idiopathic problem. Initial reports demonstrated possible associations of painful ejaculation with calculi in the seminal vesicles [588], sexual neurasthenia [589], sexually transmitted diseases [587, 590], inflammation of the prostate [591, 592], PCa [593, 594], BPH [50], prostate surgery [595, 596], pelvic radiation [597], herniorrhaphy [598] and antidepressants [599–601]. Further case reports have suggested that mercury toxicity or Ciguatera toxin fish poisoning may also result in painful ejaculation [602, 603]. Psychological issues may also be the cause of painful ejaculation, especially if the patient does not experience this problem during
- Investigation and treatment
Treatment of painful ejaculation must be tailored according to the underlying cause, if detected. Psychotherapy or relationship counselling, withdrawal of suspected agents (drugs, toxins, or radiation) [599, 600, 604] or the prescription of appropriate medical treatment (antibiotics, α-blockers or anti-inflammatory agents) may ameliorate painful ejaculation. Behavioural therapy, muscle relaxants, antidepressant treatment, anticonvulsant drugs and/or opioids, pelvic floor exercises, may be implemented if no underlying cause can be identified [605, 606].
- Surgical intervention
If medical treatments fail, surgical operations such as TURP, transurethral resection of the ejaculatory duct and neurolysis of the pudendal nerve have been suggested [607, 608]. However, there is no strong supporting evidence that surgical therapy improves painful ejaculation and therefore it must be used with caution.
Retrograde ejaculation:
- Definition and classification
Retrograde ejaculation is the total, or sometimes partial, absence of antegrade ejaculation, as a result of semen passing backwards through the bladder neck into the bladder. Patients may experience a normal, or decreased, orgasmic sensation. The causes of retrograde ejaculation can be divided into neurogenic, pharmacological, urethral, or bladder neck incompetence [587].
Retrograde ejaculation:
- Definition and classification
Retrograde ejaculation is the total, or sometimes partial, absence of antegrade ejaculation, as a result of semen passing backwards through the bladder neck into the bladder. Patients may experience a normal, or decreased, orgasmic sensation. The causes of retrograde ejaculation can be divided into neurogenic, pharmacological, urethral, or bladder neck incompetence [587].
Pathophysiology and risk factors:
The process of ejaculation requires complex co-ordination and interplay between the epididymis, vas deferens, prostate, seminal vesicles, bladder neck and bulbourethral glands [609]. Upon ejaculation, sperm are rapidly conveyed along the vas deferens and into the urethra via the ejaculatory ducts. From there, the semen progresses in an antegrade fashion, in part maintained by coaptation of the bladder neck and rhythmic contractions of the periurethral muscles, co-ordinated by a centrally mediated reflex [609]. Closure of the bladder neck and seminal emission are initiated via the sympathetic nervous system from the lumbar sympathetic ganglia and subsequently hypogastric nerve. Prostatic and seminal vesicle secretion, as well as contraction of the bulbo-cavernosal, ischio-cavernosal and pelvic floor muscles are initiated by the S 2-4 parasympathetic nervous system via the pelvic nerve [609]. Any factor that disrupts this reflex and inhibits contraction of the bladder neck (internal vesical sphincter) may lead to retrograde passage of semen into the bladder. These can be broadly categorised as pharmacological, neurogenic, anatomic and endocrinal causes of retrograde ejaculation (Table 13).
Table 13: Aetiology of retrograde ejaculation [569]

- Disease management
Medical and surgical strategies exist for the treatment of retrograde ejaculation. In recent years the reliance on medical treatment as first-line management has become common practice.
Pharmacological Sympathomimetics stimulate the release of noradrenaline as well as activating α- and β-adrenergic receptors, resulting in closure of the internal urethral sphincter, restoring the antegrade flow of semen. The most common sympathomimetics are synephrine, pseudoephedrine hydrochloride, ephedrine, phenylpropanolamine and midodrine [610]. Unfortunately, as time progresses their effect diminishes [611]. Many of the studies published about the efficacy of sympathomimetics in the treatment of retrograde ejaculation suffer from small sample size, with some represented by case reports.
The use of antimuscarinics has been described, including brompheniramine maleate and imipramine, as well as in combination with sympathomimetics. The calculated efficacy of antimuscarinics alone or in combination with sympathomimetics is 22% and 39%, respectively [35]. Combination therapy appears to be more effective although statistical analysis is not yet possible due to the small sample sizes.
- Management of infertility
Infertility has been the major concern of patients with retrograde ejaculation. Beyond the use of standard sperm-retrieval techniques, such as testicular sperm extraction (TESE), three different methods of sperm acquisition have been identified for the management of infertility in patients with retrograde ejaculation. These include; i) centrifugation and resuspension of post-ejaculatory urine specimens; ii) the Hotchkiss (or modified Hotchkiss) technique; and, iii) ejaculation on a full bladder.
1. Centrifugation and resuspension. In order to improve the ambient conditions for the sperm, the patient is asked to increase their fluid intake or take sodium bicarbonate to dilute or alkalise the urine, respectively. Afterwards, a post-orgasmic urine sample is collected by introducing a catheter or spontaneous voiding. This sample is then centrifuged and suspended in a medium. The types of suspension fluids are heterogeneous and can include bovine serum albumin, human serum albumin, Earle’s/Hank’s balanced salt solution and the patient’s urine. The resultant modified sperm mixture can then be used in assisted reproductive techniques. A systematic review of studies in couples in which male partner had retrograde ejaculation found a 15% pregnancy rate per cycle (0-100%) [35].
2. Hotchkiss method. The Hotchkiss method involves emptying the bladder prior to ejaculation, using a catheter, and then washing out and instilling a small quantity of Lactated Ringers to improve the ambient condition of the bladder. The patient then ejaculates, and semen is retrieved by catheterisation or voiding [612]. Modified Hotchkiss methods involve variance in the instillation medium. Pregnancy rates were 24% per cycle (0-100%) [35].
3. Ejaculation on a full bladder. Few papers have described results using this technique [613, 614]. The patient is encouraged to ejaculate on a full bladder and semen is suspended in Baker’s Buffer. The pregnancy rate in the two studies, which included only five patients in total, was 60% [35].
Anorgasmia:
- Definition and classification
Anorgasmia is the perceived absence of orgasm and can give rise to anejaculation. Regardless of the presence of ejaculation, anorgasmia can be a lifelong (primary) or acquired (secondary) disorder [32].
- Pathophysiology and risk factors
Primary anorgasmia is defined as starting from a man’s first sexual intercourse and lasts throughout his life, while for secondary anorgasmia patients should have a normal period before the problem starts [615]. Substance abuse, obesity and some non-specific psychological aspects, such as anxiety and fear, are considered risk factors for anorgasmia. Only a few studies have described anorgasmia alone and generally it has been considered as a symptom linked to ejaculatory disorders especially with DE, and therefore, they are believed to share the same risk factors. However, psychological factors are considered to be responsible for 90% of anorgasmia problems [616]. Causes of delayed orgasm and anorgasmia are shown in Table 14 [615].
Table 14: Causes of delayed orgasm and anorgasmia
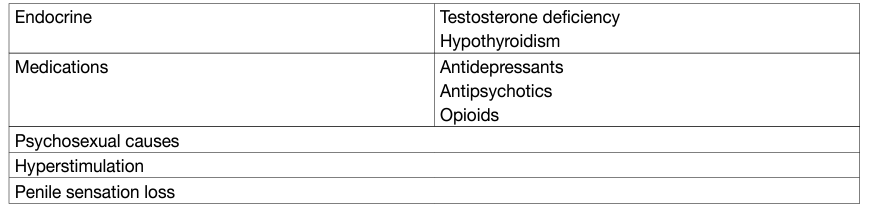
Disease management:
The psychological/behavioural strategies for anorgasmia are similar to those for DE. The patient and his partner should be examined physically and psychosexually in detail, including determining the onset of anorgasmia, medication and disease history, penile sensitivity and psychological issues. Adjunctive laboratory tests can also be used to rule out organic causes, such as testosterone, prolactin and TSH levels. Patients who have loss of penile sensitivity require further investigations [615].
- Psychological/behavioural strategies
Lifestyle changes can be recommended to affected individuals including: changing masturbation style; taking steps to improve intimacy, and decreasing alcohol consumption. Several psychotherapy techniques or their combinations have been offered, including alterations in arousal methods, reduction of sexual anxiety, roleplaying an exaggerated orgasm and increased genital stimulation [617, 618]. However, it is difficult to determine the success rates from the literature.
- Pharmacotherapy
Several drugs have been reported to reverse anorgasmia, including cyproheptadine, yohimbine, buspirone, amantadine and oxytocin [619–624]. However, these reports are generally from case-cohort studies and drugs have limited efficacy and significant adverse effect profiles. Therefore, current evidence is not strong enough to recommend drugs to treat anorgasmia.
- Management of infertility
If patients fail the treatment methods mentioned above, penile vibratory stimulation, electro-ejaculation or TESE are the choice of options for sperm retrieval in anorgasmia cases [615].
Haemospermia:
- Definition and classification
Haemospermia is defined as the appearance of blood in the ejaculate. Although it is often regarded as a symptom of minor significance, blood in the ejaculate causes anxiety in many men and may be indicative of underlying pathology [55].
- Pathophysiology and risk factors
Several causes of haemospermia have been acknowledged and can be classified into the following subcategories; idiopathic, congenital malformations, inflammatory conditions, obstruction, malignancies, vascular abnormalities, iatrogenic/trauma and systemic causes (Table 15) [625].
Table 15: Pathology associated with haemospermia
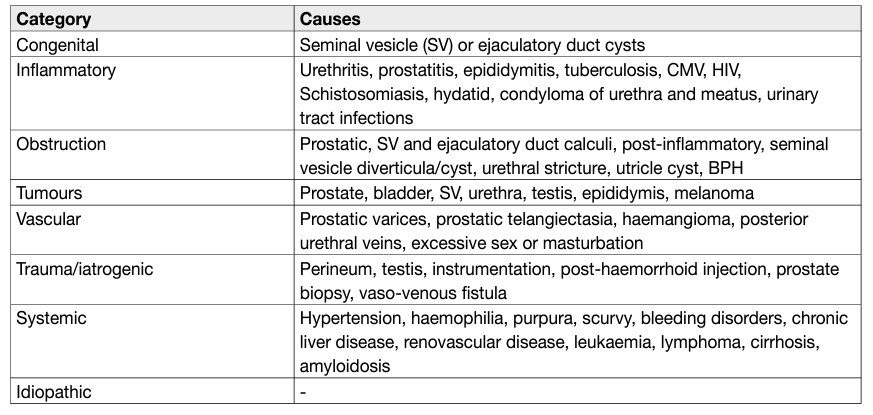
The risk of any malignancy in patients presenting with haemospermia is approximately 3.5% (0-13.1%) [626]. In an observational study of 300 consecutive patients over a 30-month period, 81% had no identified cause of haemospermia. In those patients for whom a cause was identified, the diagnosis varied dependent upon the age of presentation. When the patients were divided into those under and those over 40 years of age, UTIs were more common among younger compared to older patients (15% vs. 10.3%). In the older group (> 40 years), stones (2.2% vs. 1.4%) and malignancy (6.2% vs. 1.4%) were more common when compared with the younger cohort. In the > 40 years group, 13 patients had PCa and one had low-grade urethral carcinoma. In the < 40 years group, one patient had testicular cancer [54]. In a recent study in which 342 patients with haemospermia were included, the most relevant aetiology for haemospermia was inflammation/infection (49.4%) while genitourinary cancers (i.e., prostate and testis) only accounted for 3.2% of the cases [627].
- Investigations
As with other clinical conditions, a systematic clinical history and assessment to help identify the cause of haemospermia is undertaken. Although the differential diagnosis is extensive, most cases are caused by infections or other inflammatory processes [55]. The basic examination of haemospermia should start with a thorough symptom-specific and systemic clinical history. The first step is to understand if the patient has true haemospermia. Pseudo-haemospermia may occur as a consequence of haematuria or even suction of a partner’s blood into the urethra during copulation [587, 628, 629]. A sexual history should be taken to identify those whose haemospermia may be a consequence of a sexually transmitted disease. Recent foreign travel to areas affected by schistosomiasis or tuberculosis should also be considered. The possibility of co-existing systemic disease such as hypertension, liver disease and coagulopathy should be investigated along with systemic features of malignancy such as weight loss, loss of appetite or bone pain. Examination of the patient should also include measurement of blood pressure, as there have been several case reports suggesting an association between uncontrolled hypertension and haemospermia [630, 631]. Most authors who propose an investigative baseline agree on the initial diagnostic tests, but there is no consensus in this regard [625, 626, 628]. Urinalysis should be performed along with sending the urine for culture and sensitivity testing, as well as microscopy. If tuberculosis or schistosomiasis is the suspected cause, the semen or prostatic secretions should be sent for analysis. A full sexually-transmitted disease screen, including first-void urine as well as serum and genitourinary samples, should be tested for Chlamydia, Ureaplasma and Herpes Simplex virus. Using this strategy, it may be possible to find an infectious agent among cases that would have been labelled as idiopathic haemospermia [632]. Serum PSA should be taken in men aged > 40 years who have been appropriately counselled [56]. Blood work including a full blood count, liver function tests, and a clotting screen should be taken to identify systemic diseases. The question of whether further investigation is warranted depends on clinician judgment, patient age and an assessment of risk factors [625]. Digital rectal examination (DRE) should also be performed and the meatus re-examined after DRE for bloody discharge [633]. Detection of a palpable nodule in the prostate is important because an association between haemospermia and PCa has been postulated although not completely proven. Magnetic resonance imaging is being increasingly used as a definitive means to investigate haemospermia. The multiplanar ability of MRI to accurately represent structural changes in the prostate, seminal vesicles, ampulla of vas deferens, and ejaculatory ducts has enabled the technique to be particularly useful in determining the origin of midline or paramedian prostatic cysts and in determining optimal surgical management [634]. The addition of an endorectal coil can improve the diagnostic accuracy for identifying the site and possible causes of haemorrhage [635]. Cystoscopy has been included in most suggested investigative protocols in patients with high-risk features (patients who are refractory to conservative treatment and who have persistent haemospermia). It can provide invaluable information as it allows direct visualisation of the main structures in the urinary tract that can be attributed to causes of haemospermia, such as: polyps, urethritis, prostatic cysts, foreign bodies, calcifications and vascular abnormalities [636, 637]. With the advancement of optics, the ability to create ureteroscopes of diameters small enough to allow insertion into the ejaculatory duct and seminal vesicles has been made possible [638]. In a prospective study, 106 patients with prolonged haemospermia underwent transrectal US and seminal vesiculoscopy. With both methods combined, diagnosis was made in 87.7% of patients. When compared head-to-head, the diagnostic yield for TRUS vs. seminal vesiculoscopy was 45.3% and 74.5%, respectively (P < 0.001) [639]. Melanospermia is a consequence of malignant melanoma involving the genitourinary tract and is a rare condition that has been described in two case reports [640, 641]. Chromatography of the semen sample can be used to distinguish the two by identifying the presence of melanin if needed.
- Disease management
Conservative management is generally the primary treatment option when the patients are aged < 40 years and have a single episode of haemospermia. The primary goal of treatment is to exclude malignant conditions like prostate and bladder cancer and treat any other underlying cause. If no pathology is found, then the patient can be reassured [55, 625]. Patients with recurrent haemospermia who are middle-aged, warrant more aggressive intervention. Appropriate antibiotic therapy should be given to patients who have urogenital infections or STIs. Urethral or prostate varices or angiodyplastic vessels can be fulgurated, whereas cysts, either of the seminal vesicles or prostatic urethra, can be aspirated transrectally [55]. Ejaculatory duct obstruction is managed by transurethral incision at the duct opening [642, 643]. Systemic conditions should be treated appropriately [626, 629, 644, 645]. Defining a management algorithm for haemospermia is based on the patient age and degree of haemospermia. Patients often find blood in the ejaculate alarming, and investigations should be aimed at excluding a serious, despite infrequent, underlying cause (e.g., cancer), while at the same time preventing over-investigation and alleviating patient anxiety. The literature describes a multitude of causes for haemospermia, although many of these are not commonly found after investigation. However, men may be stratified into higher-risk groups according to several factors including: age > 40 years, recurrent or persistent haemospermia, actual risk for PCa (e.g., positive family history), and concurrent haematuria. Based upon the literature, a management algorithm is proposed (Figure 6) [626, 629, 644, 645].
Figure 6: Management algorithm for haemospermia
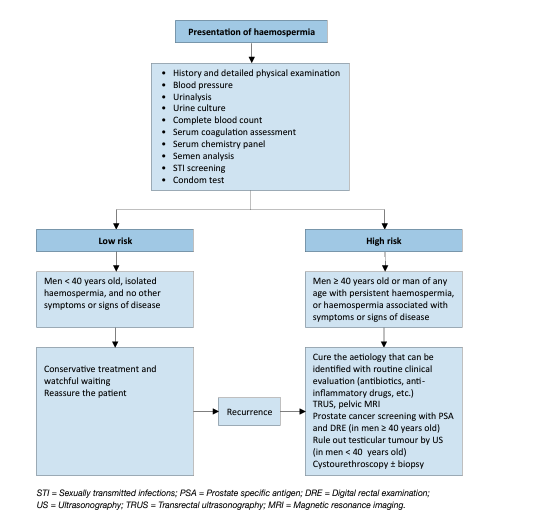
REFERENCES:
- Eardley, I. The Incidence, Prevalence, and Natural History of Erectile Dysfunction. Sex Med Rev, 2013. 1: 3.
- Feldman, H.A., et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol, 1994. 151: 54.
- Braun, M., et al. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res, 2000. 12: 305.
- Johannes, C.B., et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol, 2000. 163: 460.
- Schouten, B.W., et al. Incidence rates of erectile dysfunction in the Dutch general population. Effects of definition, clinical relevance and duration of follow-up in the Krimpen Study. Int J Impot Res, 2005. 17: 58.
- Capogrosso, P., et al. One patient out of four with newly diagnosed erectile dysfunction is a young man-worrisome picture from the everyday clinical practice. J Sex Med, 2013. 10: 1833.
- Waldinger, M.D. The neurobiological approach to premature ejaculation. J Urol, 2002. 168: 2359.
- Althof, S.E., et al. International Society for Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med, 2010. 7: 2947.
- Hatzimouratidis, K., et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol, 2010. 57: 804.
- McMahon, C.G., et al. An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation. J Sex Med, 2008. 5: 1590.
- Carson, C., et al. Premature ejaculation: definition and prevalence. Int J Impot Res, 2006. 18 Suppl 1: S5.
- Laumann, E.O., et al. Sexual dysfunction in the United States: prevalence and predictors. JAMA, 1999. 281: 537.
- Porst, H., et al. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol, 2007. 51: 816.
- Serefoglu, E.C., et al. Prevalence of the complaint of ejaculating prematurely and the four premature ejaculation syndromes: results from the Turkish Society of Andrology Sexual Health Survey. J Sex Med, 2011. 8: 540.
- Gao, J., et al. Prevalence and factors associated with the complaint of premature ejaculation and the four premature ejaculation syndromes: a large observational study in China. J Sex Med, 2013. 10: 1874.
- Waldinger, M.D., et al. The use of old and recent DSM definitions of premature ejaculation in observational studies: a contribution to the present debate for a new classification of PE in the DSM-V. J Sex Med, 2008. 5: 1079.
- Serefoglu, E.C., et al. The comparison of premature ejaculation assessment questionnaires and their sensitivity for the four premature ejaculation syndromes: results from the Turkish society of andrology sexual health survey. J Sex Med, 2011. 8: 1177.
- Serefoglu, E.C., et al. The distribution of patients who seek treatment for the complaint of ejaculating prematurely according to the four premature ejaculation syndromes. J Sex Med, 2010. 7: 810.
- Zhang, X., et al. Distribution and factors associated with four premature ejaculation syndromes in outpatients complaining of ejaculating prematurely. J Sex Med, 2013. 10: 1603.
- Althof, S.E., et al. An update of the International Society of Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE). J Sex Med, 2014. 11: 1392.
- Perelman, M. Retarded Ejaculation. Curr Sex Hlth Rep, 2004. 1: 95.
- Simons, J.S., et al. Prevalence of sexual dysfunctions: results from a decade of research. Arch Sex Behav, 2001. 30: 177.
- Mercer, C.H., et al. Who reports sexual function problems? Empirical evidence from Britain’s 2000 National Survey of Sexual Attitudes and Lifestyles. Sex Transm Infect, 2005. 81: 394.
- Laumann, E.O., et al. Sexual problems among women and men aged 40-80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res, 2005. 17: 39.
- Lindau, S.T., et al. A study of sexuality and health among older adults in the United States. N Engl J Med, 2007. 357: 762.
- Perelman, M.A. Regarding ejaculation: delayed and otherwise [letter to the editor]. J Androl., 2003. 24: 496.
- Perelman, M.A., et al., Evaluation and Treatment of Ejaculatory Disorders, In: Atlas of Male Sexual Dysfunction, T.F. Lue, Editor. 2004, Current Medicine LLC: Philadelphia.
- Perelman, M.A., et al. Retarded ejaculation. World J Urol, 2006. 24: 645.
- Verma, K.K., et al. The frequency of sexual dysfunctions in patients attending a sex therapy clinic in north India. Arch Sex Behav, 1998. 27: 309.
- Nazareth, I., et al. Problems with sexual function in people attending London general practitioners: cross sectional study. BMJ, 2003. 327: 423.
- DSM, V., American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 2013, Arlington, VA [access date: 1 June 2013].
- Di Sante, S., et al. Epidemiology of delayed ejaculation. Transl Androl Urol, 2016. 5: 541.
- Kinsey, A.C., et al. Sexual behavior in the human male. 1948. Am J Public Health, 2003. 93: 894.
- Chehensse, C., et al. The spinal control of ejaculation revisited: a systematic review and meta-analysis of anejaculation in spinal cord injured patients. Hum Reprod Update, 2013. 19: 507.
- Jefferys, A., et al. The management of retrograde ejaculation: a systematic review and update. Feril Steril, 2012. 97: 306.
- Gandhi, J., et al. The Role of Diabetes Mellitus in Sexual and Reproductive Health: An Overview of Pathogenesis, Evaluation, and Management. Curr Diabetes Rev, 2017. 13: 573.
- Yavetz, H., et al. Retrograde ejaculation. Hum Reprod, 1994. 9: 381.
- Fedder, J., et al. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology, 2013. 1: 602.
- Hylmarova, S., et al. The impact of type 1 diabetes mellitus on male sexual functions and sex hormone levels. Endocr J, 2020. 67: 59.
- Emberton, M., et al. The effect of prostatectomy on symptom severity and quality of life. Br J Urol, 1996. 77: 233.
- Woo, H.H., et al. Preservation of sexual function with the prostatic urethral lift: a novel treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med, 2012. 9: 568.
- Talab, S.S., et al. V403: The impact of Ejaculation-Preserving Photo-selective Vaporization of the Prostate (EP-PVP) on lower urinary tract symptoms and ejaculatory function: results of a multicenter study. J Urol, 2013.
- Liu, Y., et al. Impact on Sexual Function of Endoscopic Enucleation vs Transurethral Resection of the Prostate for Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis. J Endourol, 2020. 34: 1064.
- Suarez-Ibarrola, R., et al. Efficacy and safety of aquablation of the prostate for patients with symptomatic benign prostatic enlargement: a systematic review. World J Urol, 2020. 38: 1147.
- Bebi, C., et al. Sexual and ejaculatory function after holmium laser enucleation of the prostate and bipolar transurethral enucleation of the prostate: a single-center experience. Int J Impot Res, 2020.
- Lindal, E., et al. The lifetime prevalence of psychosexual dysfunction among 55 to 57-year-olds in Iceland. Soc Psychiatry Psychiatr Epidemiol, 1993. 28: 91.
- Blanker, M.H., et al. Erectile and ejaculatory dysfunction in a community-based sample of men 50 to 78 years old: prevalence, concern, and relation to sexual activity. Urology, 2001. 57: 763.
- Roberts, R.O., et al. Prevalence of prostatitis-like symptoms in a community based cohort of older men. J Urol, 2002. 168: 2467.
- Sonmez, N.C., et al. Sexual dysfunction in type III chronic prostatitis (CP) and chronic pelvic pain syndrome (CPPS) observed in Turkish patients. Int Urol Nephrol, 2011. 43: 309.
- Nickel, J.C., et al. Benign prostatic hyperplasia (BPH) and prostatitis: prevalence of painful ejaculation in men with clinical BPH. BJU Int, 2005. 95: 571.
- Mo, M.Q., et al. Sexual dysfunctions and psychological disorders associated with type IIIa chronic prostatitis: a clinical survey in China. Int Urol Nephrol, 2014. 46: 2255.
- Wagenlehner, F.M., et al. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur Urol, 2013. 63: 953.
- Shoskes, D.A., et al. Impact of post-ejaculatory pain in men with category III chronic prostatitis/chronic pelvic pain syndrome. J Urol, 2004. 172: 542.
- Ng, Y.H., et al. Haematospermia as a presenting symptom: outcomes of investigation in 300 men. Surgeon, 2013. 11: 35.
- Mulhall, J.P., et al. Hemospermia: diagnosis and management. Urology, 1995. 46: 463.
- Han, M., et al. Association of hemospermia with prostate cancer. J Urol, 2004. 172: 2189.
- Fugl-Meyer, A., et al. Sexual disabilities, problems and satisfaction in 18-74 year old Swedes. Scand J Sexol, 1999. 2: 79.
- Quinta Gomes, A.L., et al. Prevalence of sexual problems in Portugal: results of a population-based study using a stratified sample of men aged 18 to 70 years. J Sex Res, 2014. 51: 13.
- Martin, S., et al. Clinical and biopsychosocial determinants of sexual dysfunction in middle-aged and older Australian men. J Sex Med, 2012. 9: 2093.
- Hirshfield, S., et al. Sexual Dysfunction in an Internet Sample of U.S. Men Who Have Sex with Men. J Sex Med, 2010. 7: 3104.
- Peixoto, M.M., et al. Prevalence of sexual problems and associated distress among gay and heterosexual men. Sex Relat Ther, 2015. 30: 211.
- Najman, J.M., et al. Sexual dysfunction in the Australian population. Aust Fam Physician, 2003. 32: 951.
- Traeen, B., et al. Sexual problems in 18-67-year-old Norwegians. Scand J Public Health, 2010. 38: 445.
- Gratzke, C., et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med, 2010. 7: 445.
- NIH, C.D.P.o.I. NIH Consensus Conference. Impotence. JAMA, 1993. 270: 83.
- Fisher, W.A., et al. Erectile dysfunction (ED) is a shared sexual concern of couples I: couple conceptions of ED. J Sex Med, 2009. 6: 2746.
- Salonia, A., et al. Is erectile dysfunction a reliable proxy of general male health status? The case for the International Index of Erectile Function-Erectile Function domain. J Sex Med, 2012. 9: 2708.
- Corona, G., et al. Assessment of the relational factor in male patients consulting for sexual dysfunction: the concept of couple sexual dysfunction. J Androl, 2006. 27: 795.
- Vlachopoulos, C.V., et al. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes, 2013. 6: 99.
- Dong, J.Y., et al. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol, 2011. 58: 1378.
- Gandaglia, G., et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol, 2014. 65: 968.
- Zhao, B., et al. Erectile Dysfunction Predicts Cardiovascular Events as an Independent Risk Factor: A Systematic Review and Meta-Analysis. J Sex Med, 2019. 16: 1005.
- Pozzi, E., et al. Longitudinal Risk of Developing Cardiovascular Diseases in Patients With Erectile Dysfunction Which Patients Deserve More Attention? J Sex Med, 2020. 17: 1489.
- Pastuszak, A.W., et al. Erectile dysfunction as a marker for cardiovascular disease diagnosis and intervention: a cost analysis. J Sex Med, 2015. 12: 975.
- Besiroglu, H., et al. The relationship between metabolic syndrome, its components, and erectile dysfunction: a systematic review and a meta-analysis of observational studies. J Sex Med, 2015. 12: 1309.
- Jackson, G., et al. Cardiovascular aspects of sexual medicine. J Sex Med, 2010. 7: 1608.
- Binmoammar, T.A., et al. The impact of poor glycaemic control on the prevalence of erectile dysfunction in men with type 2 diabetes mellitus: a systematic review. JRSM Open, 2016. 7: 2054270415622602.
- Glina, F.P.A., et al. What Is the Impact of Bariatric Surgery on Erectile Function? A Systematic Review and MetaAnalysis. Sex Med Rev, 2017. 5: 393.
- Sansone, A., et al. Serum Homocysteine Levels in Men with and without Erectile Dysfunction: A Systematic Review and Meta-Analysis. Int J Endocrinol, 2018. 2018: 7424792.
- Corona, G., et al. Sexual dysfunction at the onset of type 2 diabetes: the interplay of depression, hormonal and cardiovascular factors. J Sex Med, 2014. 11: 2065.
- Pizzol, D., et al. Associations between body mass index, waist circumference and erectile dysfunction: a systematic review and META-analysis. Rev Endocr Metab Disord, 2020. 21: 657.
- Sivaratnam, L., et al. Behavior-Related Erectile Dysfunction: A Systematic Review and Meta-Analysis. J Sex Med, 2021. 18: 121.
- Alberti, L., et al. Erectile dysfunction in heart failure patients: a critical reappraisal. Andrology, 2013. 1: 177.
- Baumhakel, M., et al. Cardiovascular risk, drugs and erectile function–a systematic analysis. Int J Clin Pract, 2011. 65: 289.
- Trinchieri, M., et al. Erectile and Ejaculatory Dysfunction Associated with Use of Psychotropic Drugs: A Systematic Review. J Sex Med, 2021. 18: 1354.
- Lin, W.Y., et al. Atrial fibrillation is associated with increased risk of erectile dysfunction: A nationwide populationbased cohort study. Int J Cardiol, 2015. 190: 106.
- Corona, G., et al. Endocrinologic Control of Men’s Sexual Desire and Arousal/Erection. J Sex Med, 2016. 13: 317.
- Farag, Y.M., et al. Vitamin D deficiency is independently associated with greater prevalence of erectile dysfunction: The National Health and Nutrition Examination Survey (NHANES) 2001-2004. Atherosclerosis, 2016. 252: 61.
- Caretta, N., et al. Hypovitaminosis D is associated with erectile dysfunction in type 2 diabetes. Endocrine, 2016. 53: 831.
- Salem, S., et al. Serum uric acid as a risk predictor for erectile dysfunction. J Sex Med, 2014. 11: 1118.
- Zhang, Y., et al. Serum Folic Acid and Erectile Dysfunction: A Systematic Review and Meta-Analysis. Sex Med, 2021. 9: 100356.
- Liu, Q., et al. Erectile Dysfunction and Depression: A Systematic Review and Meta-Analysis. J Sex Med, 2018. 15: 1073.
- Velurajah, R., et al. Erectile dysfunction in patients with anxiety disorders: a systematic review. Int J Impot Res, 2021.
- Perez-Garcia, L.F., et al. Sexual function and reproduction can be impaired in men with rheumatic diseases: A systematic review. Semin Arthritis Rheum, 2020. 50: 557.
- Zhao, S., et al. Significant Increase of Erectile Dysfunction in Men With Post-stroke: A Comprehensive Review. Front Neurol, 2021. 12: 671738.
- Luo, L., et al. Association between chronic obstructive pulmonary disease and risk of erectile dysfunction: a systematic review and meta-analysis. Int J Impot Res, 2020. 32: 159.
- Xu, J., et al. Risk of osteoporosis in patients with erectile dysfunction: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore), 2021. 100: e26326.
- Pyrgidis, N., et al. Renal Transplantation Improves Erectile Function in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. J Urol, 2021. 205: 1009.
- Rahman, I.A., et al. Effects of renal transplantation on erectile dysfunction: a systematic review and metaanalysis. Int J Impot Res, 2021.
- Hsu, C.Y., et al. Gout is associated with organic and psychogenic erectile dysfunction. Eur J Intern Med, 2015. 26: 691.
- Kellesarian, S.V., et al. Association between obstructive sleep apnea and erectile dysfunction: a systematic review and meta-analysis. Int J Impot Res, 2018. 30: 129.
- Molina Leyva, A., et al. Sexual dysfunction in psoriasis: a systematic review. [Review]. J Eur Acad Dermatol Venereol, 2015. 29: 649.
- Ji, S., et al. Erectile dysfunction in patients with plaque psoriasis: the relation of depression and cardiovascular factors. Int J Impot Res, 2016. 28: 96.
- Egeberg, A., et al. Erectile Dysfunction in Male Adults With Atopic Dermatitis and Psoriasis. J Sex Med, 2017. 14: 380.
- Fan, D., et al. Male sexual dysfunction and ankylosing spondylitis: a systematic review and metaanalysis. J Rheumatol, 2015. 42: 252.
- Duman, D.G., et al. Nonalcoholic Fatty Liver Disease is Associated with Erectile Dysfunction: A Prospective Pilot Study. J Sex Med, 2016. 13: 383.
- Kim, M., et al. Erectile dysfunction in patients with liver disease related to chronic hepatitis B. Clin Mol Hepatol, 2015. 21: 352.
- Liu, L.H., et al. Chronic periodontitis and the risk of erectile dysfunction: a systematic review and meta-analysis. Int J Impot Res, 2017. 29: 43.
- Law, G., et al. Correlation in Severity Between Glaucoma and Erectile Dysfunction. J Glaucoma, 2016. 25: 716.
- Kao, C.C., et al. Association Between Inflammatory Bowel Disease and Erectile Dysfunction: A Nationwide Population-Based Study. Inflamm Bowel Dis, 2016. 22: 1065.
- Chao, C.H., et al. Increased risk of organic erectile dysfunction in patients with chronic fatigue syndrome: a nationwide population-based cohort study. Andrology, 2015. 3: 666.
- Su, V.Y., et al. Allergic rhinitis and risk of erectile dysfunction–a nationwide population-based study. Allergy, 2013. 68: 440.
- Hughes, T.L., et al. Sexual Function and Dysfunction in Individuals with Spina Bifida: A Systematic Review. Urology, 2021. 156: 308.
- Duran, M.B., et al. Variations in the Number of Patients Presenting with Andrological Problems During the COVID-19 Pandemic and the Possible Reasons for These Variations: A Multi-Center Study. Sex Med, 2020: 100292.
- Sansone, A., et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest, 2020: 1.
- Gan, Z.S., et al. Systematic Review and Meta-Analysis of Cycling and Erectile Dysfunction. Sex Med Rev, 2021. 9: 304.
- Rovere, G., et al. Epidemiology and aetiology of male and female sexual dysfunctions related to pelvic ring injuries: a systematic review. Int Orthop, 2021. 45: 2687.
- Schmid, F.A., et al. Erectile dysfunction and penile rehabilitation after pelvic fracture: a systematic review and meta-analysis. BMJ Open, 2021. 11: e045117.
- Chew, P.Y., et al. The Association Between Female Sexual Dysfunction and Sexual Dysfunction in the Male Partner: A Systematic Review and Meta-Analysis. J Sex Med, 2021. 18: 99.
- Seftel, A.D., et al. Coexisting lower urinary tract symptoms and erectile dysfunction: a systematic review of epidemiological data. Int J Clin Pract, 2013. 67: 32.
- Rosen, R., et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol, 2003. 44: 637.
- Light, A., et al. Erectile Function Following Surgery for Benign Prostatic Obstruction: A Systematic Review and Network Meta-analysis of Randomised Controlled Trials. Eur Urol, 2021. 80: 174.
- Verze, P., et al. The impact of surgery for lower urinary tract symptoms/benign prostatic enlargement on both erectile and ejaculatory function: a systematic review. Int J Impot Res, 2019.
- Li, H.J., et al. Prevalence of sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: a meta-analysis. World J Urol, 2016. 34: 1009.
- Chung, S.D., et al. A nationwide population-based study on bladder pain syndrome/interstitial cystitis and ED. Int J Impot Res, 2013. 25: 224.
- Corona, G., et al. Interplay Between Premature Ejaculation and Erectile Dysfunction: A Systematic Review and Meta-Analysis. J Sex Med, 2015. 12: 2291.
- Fainberg, J., et al. Erectile Dysfunction is a Transient Complication of Prostate Biopsy: A Systematic Review and Meta-Analysis. J Urol, 2021. 205: 664.
- Murray, K.S., et al. A prospective study of erectile function after transrectal ultrasonography-guided prostate biopsy. BJU Int, 2015. 116: 190.
- Feng, C., et al. The relationship between erectile dysfunction and open urethroplasty: a systematic review and meta-analysis. J Sex Med, 2013. 10: 2060.
- Redmond, E., et al. Comprehensive Prospective Assessment of Patient-reported Outcomes Following Urethroplasty. Urology, 2020. 141: 162.
- D’Hulst, P., et al. Patient-reported outcomes after buccal mucosal graft urethroplasty for bulbar urethral strictures: results of a prospective single-centre cohort study. BJU Int, 2020. 126: 684.
- Gupta, B.P., et al. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med, 2011. 171: 1797.
- Gerbild, H., et al. Physical Activity to Improve Erectile Function: A Systematic Review of Intervention Studies. Sex Med, 2018. 6: 75.
- Collins, C.E., et al. Improvement in erectile function following weight loss in obese men: the SHED-IT randomized controlled trial. Obes Res Clin Pract, 2013. 7: e450.
- Glina, S., et al. Modifying risk factors to prevent and treat erectile dysfunction. J Sex Med, 2013. 10: 115.
- Vlachopoulos, C., et al. Erectile dysfunction in the cardiovascular patient. Eur Heart J, 2013. 34: 2034.
- Cui, Y., et al. The effect of statins on erectile dysfunction: a systematic review and meta-analysis. J Sex Med, 2014. 11: 1367.
- Cai, X., et al. The role of statins in erectile dysfunction: a systematic review and meta-analysis. Asian J Androl, 2014. 16: 461.
- Emanu, J.C., et al. Erectile dysfunction after radical prostatectomy: prevalence, medical treatments, and psychosocial interventions. Curr Opin Support Palliat Care, 2016. 10: 102.
- Modh, R.A., et al. Sexual dysfunction after cystectomy and urinary diversion. Nat Rev Urol, 2014. 11: 445.
- Celentano, V., et al. Sexual dysfunction following rectal cancer surgery. Int J Colorectal Dis, 2017. 32: 1523.
- Walz, J., et al. A Critical Analysis of the Current Knowledge of Surgical Anatomy of the Prostate Related to Optimisation of Cancer Control and Preservation of Continence and Erection in Candidates for Radical Prostatectomy: An Update. Eur Urol, 2016. 70: 301.
- Capogrosso, P., et al. Erectile Recovery After Radical Pelvic Surgery: Methodological Challenges and Recommendations for Data Reporting. J Sex Med, 2019.
- Salonia, A., et al. Sexual Rehabilitation After Treatment For Prostate Cancer-Part 2: Recommendations From the Fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med, 2017. 14: 297.
- Hunt, A.A., et al. Risk of erectile dysfunction after modern radiotherapy for intact prostate cancer. Prostate Cancer Prostatic Dis, 2020.
- Nolsøe, A.B., et al. Neglected side effects to curative prostate cancer treatments. Int J Impot Res, 2020.
- Maggi, M., et al. Psychological impact of different primary treatments for prostate cancer: A critical analysis. Andrologia, 2019. 51: e13157.
- Fenton, J.J., et al. Prostate-Specific Antigen-Based Screening for Prostate Cancer: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA, 2018. 319: 1914.
- Lardas, M., et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur Urol, 2017. 72: 869.
- Mottet, N., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol, 2016.
- Salonia, A., et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol, 2012. 62: 261.
- Boyle, H.J., et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer, 2019. 116: 116.
- Sanda, M.G., et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med, 2008. 358: 1250.
- Tal, R., et al. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med, 2009. 6: 2538.
- Schauer, I., et al. Have rates of erectile dysfunction improved within the past 17 years after radical prostatectomy? A systematic analysis of the control arms of prospective randomized trials on penile rehabilitation. Andrology, 2015. 3: 661.
- Capogrosso, P., et al. Are We Improving Erectile Function Recovery After Radical Prostatectomy? Analysis of Patients Treated over the Last Decade. Eur Urol, 2019. 75: 221.
- Khoder, W.Y., et al. Do we need the nerve sparing radical prostatectomy techniques (intrafascial vs. interfascial) in men with erectile dysfunction? Results of a single-centre study. World J Urol, 2015. 33: 301.
- Ju, I.E., et al. Surgeon Experience and Erectile Function After Radical Prostatectomy: A Systematic Review. Sex Med Rev, 2021. 9: 650.
- Glickman, L., et al. Changes in continence and erectile function between 2 and 4 years after radical prostatectomy. J Urol, 2009. 181: 731.
- Ficarra, V., et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol, 2012. 62: 418.
- Stolzenburg, J.U., et al. Effect of surgical approach on erectile function recovery following bilateral nerve-sparing radical prostatectomy: An evaluation utilising data from a randomised, double-blind, double-dummy multicentre trial of tadalafil vs placebo. BJU International, 2015. 116: 251.
- Haglind, E., et al. Urinary Incontinence and Erectile Dysfunction after Robotic Versus Open Radical Prostatectomy: A Prospective, Controlled, Nonrandomised Trial. Eur Urol, 2015. 68: 216.
- Yaxley, J.W., et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet, 2016. 388: 1057.
- Isgoren, A., et al. Erectile function outcomes after robot-assisted radical prostatectomy: is it superior to open retropubic or laparoscopic approach? Sex Med Rev, 2014. 2: 10.
- Stember, D.S., et al. The concept of erectile function preservation (penile rehabilitation) in the patient after brachytherapy for prostate cancer. Brachytherapy, 2012. 11: 87.
- Gaither, T.W., et al. The Natural History of Erectile Dysfunction After Prostatic Radiotherapy: A Systematic Review and Meta-Analysis. J Sex Med, 2017. 14: 1071.
- Loi, M., et al. Sexual Function in Patients Treated With Stereotactic Radiotherapy For Prostate Cancer: A Systematic Review of the Current Evidence. J Sex Med, 2019. 16: 1409.
- Fallara, G., et al. Erectile function after focal therapy for localized prostate cancer: a systematic review. Int J Impot Res, 2021. 33: 418.
- Valerio, M., et al. New and Established Technology in Focal Ablation of the Prostate: A Systematic Review. Eur Urol, 2017. 71: 17.
- van der Poel, H.G., et al. Focal Therapy in Primary Localised Prostate Cancer: The European Association of Urology Position in 2018. Eur Urol, 2018. 74: 84.
- Hatzichristou, D., et al. Diagnosing Sexual Dysfunction in Men and Women: Sexual History Taking and the Role of Symptom Scales and Questionnaires. J Sex Med, 2016. 13: 1166.
- The Process of Care Consensus Panel. The process of care model for evaluation and treatment of erectile dysfunction. Int J Impot Res, 1999. 11: 59.
- Althof, S.E., et al. Standard operating procedures for taking a sexual history. J Sex Med, 2013. 10: 26.
- Rosen, R.C., et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology, 1997. 49: 822.
- Petrone, L., et al. Structured interview on erectile dysfunction (SIEDY): a new, multidimensional instrument for quantification of pathogenetic issues on erectile dysfunction. Int J Impot Res, 2003. 15: 210.
- Mulhall, J.P., et al. Validation of the erection hardness score. J Sex Med, 2007. 4: 1626.
- Beck, A.T., et al., Manual for the Beck depression inventory-II. 1996, San Antonio, TX: Psychological Corporation.
- Khera, M., et al. Diagnosis and Treatment of Testosterone Deficiency: Recommendations From the Fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med, 2016. 13: 1787.
- Davis-Joseph, B., et al. Accuracy of the initial history and physical examination to establish the etiology of erectile dysfunction. Urology, 1995. 45: 498.
- Ghanem, H.M., et al. SOP: physical examination and laboratory testing for men with erectile dysfunction. J Sex Med, 2013. 10: 108.
- Isidori, A.M., et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. Eur Urol, 2014. 65: 99.
- Buvat, J., et al. Endocrine aspects of male sexual dysfunctions. J Sex Med, 2010. 7: 1627.
- Bhasin, S., et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab, 2010. 95: 2536.
- O’Connor, D.B., et al. The relationships between sex hormones and sexual function in middle-aged and older European men. J Clin Endocrinol Metab, 2011. 96: E1577.
- Heidenreich, A., et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol, 2014. 65: 124.
- Maggi, M., et al. Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia, thyroid disorders, GH disorders, and DHEA). J Sex Med, 2013. 10: 661.
- Miner, M., et al. Cardiometabolic risk and female sexual health: the Princeton III summary. J Sex Med, 2012. 9: 641.
- Gazzaruso, C., et al. Erectile dysfunction can improve the effectiveness of the current guidelines for the screening for asymptomatic coronary artery disease in diabetes. Endocrine, 2011. 40: 273.
- Turek, S.J., et al. Sexual dysfunction as a marker of cardiovascular disease in males with 50 or more years of type 1 diabetes. Diabetes Care, 2013. 36: 3222.
- Tanaka, Y., et al. Association of Erectile Dysfunction with Incident Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Med, 2020. 133: 613.
- Vlachopoulos, C., et al. Prediction of cardiovascular events with aortic stiffness in patients with erectile dysfunction. Hypertension, 2014. 64: 672.
- Omland, T., et al. Relation of Erectile Dysfunction to Subclinical Myocardial Injury. Am J Cardiol, 2016. 118: 1821.
- Fang, S.C., et al. Changes in erectile dysfunction over time in relation to Framingham cardiovascular risk in the Boston Area Community Health (BACH) Survey. J Sex Med, 2015. 12-1.
- Nehra, A., et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc, 2012. 87: 766.
- DeBusk, R., et al. Management of sexual dysfunction in patients with cardiovascular disease: recommendations of The Princeton Consensus Panel. Am J Cardiol, 2000. 86: 175.
- Kostis, J.B., et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol, 2005. 96: 313.
- Zou, Z., et al. The Role of Nocturnal Penile Tumescence and Rigidity (NPTR) Monitoring in the Diagnosis of Psychogenic Erectile Dysfunction: A Review. Sex Med Rev, 2019. 7: 442.
- Qin, F., et al. Advantages and limitations of sleep-related erection and rigidity monitoring: a review. Int J Impot Res, 2018. 30: 192.
- Hatzichristou, D.G., et al. Hemodynamic characterization of a functional erection. Arterial and corporeal venoocclusive function in patients with a positive intracavernosal injection test. Eur Urol, 1999. 36: 60.
- Sikka, S.C., et al. Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med, 2013. 10: 120.
- Pathak, R.A., et al. Novel Evidence-Based Classification of Cavernous Venous Occlusive Disease. J Urol, 2016. 196: 1223.
- Capogrosso, P., et al. Low-Intensity Shock Wave Therapy in Sexual Medicine-Clinical Recommendations from the European Society of Sexual Medicine (ESSM). J Sex Med, 2019. 16: 1490.
- Li, K., et al. The Relationships of Dehydroepiandrosterone Sulfate, Erectile Function and General Psychological Health. Sex Med, 2021. 9: 100386.
- Nguyen, H.M.T., et al. Erectile Dysfunction in Young Men-A Review of the Prevalence and Risk Factors. Sex Med Rev, 2017. 5: 508.
- Carvalho, J., et al. The Relationship Between COVID-19 Confinement, Psychological Adjustment, and Sexual Functioning, in a Sample of Portuguese Men and Women. J Sex Med, 2021. 18: 1191.
- McCabe, M.P., et al. A systematic review of the psychosocial outcomes associated with erectile dysfunction: does the impact of erectile dysfunction extend beyond a man’s inability to have sex? J Sex Med, 2014. 11: 347.
- Rosen, R.C., et al. Men with Sexual Problems and Their Partners: Findings from the International Survey of Relationships. Arch Sex Behav, 2016. 45: 159.
- Walther, A., et al. Psychobiological Protective Factors Modifying the Association Between Age and Sexual Health in Men: Findings From the Men’s Health 40+ Study. Am J Mens Health, 2017. 11: 737.
- Tavares, I.M., et al. The Role of Cognitive Processing Factors in Sexual Function and Dysfunction in Women and Men: A Systematic Review. Sex Med Rev, 2020. 8: 403.
- Ejder Apay, S., et al. The Sexual Beliefs of Turkish Men: Comparing the Beliefs of Men With and Without Erectile Dysfunction. J Sex Marital Ther, 2015. 41: 661.
- Rowland, D.L., et al. Self-efficacy as a relevant construct in understanding sexual response and dysfunction. J Sex Marital Ther, 2015. 41: 60.
- Brotto, L., et al. Psychological and Interpersonal Dimensions of Sexual Function and Dysfunction. J Sex Med, 2016. 13: 538.
- Giuri, S., et al. Cognitive Attentional Syndrome and Metacognitive Beliefs in Male Sexual Dysfunction: An Exploratory Study. Am J Mens Health, 2017. 11: 592.
- Fruhauf, S., et al. Efficacy of psychological interventions for sexual dysfunction: a systematic review and metaanalysis. Arch Sex Behav, 2013. 42: 915.
- Montorsi, F., et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med, 2010. 7: 3572.
- Hatzichristou, D., et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med, 2010. 7: 337.
- Hatzimouratidis, K., et al. Pharmacotherapy for Erectile Dysfunction: Recommendations From the Fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med, 2016. 13: 465.
- Moyad, M.A., et al. Prevention and treatment of erectile dysfunction using lifestyle changes and dietary supplements: what works and what is worthless, part II. Urol Clin North Am, 2004. 31: 259.
- Isidori, A.M., et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. Eur Urol, 2014. 65: 99.
- Williams, P., et al. Men’s beliefs about treatment for erectile dysfunction-what influences treatment use? A systematic review. Int J Impot Res, 2020.
- Yuan, J., et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol, 2013. 63: 902.
- Salonia, A., et al. Sildenafil in erectile dysfunction: a critical review. Curr Med Res Opin, 2003. 19: 241.
- Lue, T.F. Erectile dysfunction. N Engl J Med, 2000. 342: 1802.
- Goldstein, I., et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med, 1998. 338: 1397.
- Moncada, I., et al. Efficacy of sildenafil citrate at 12 hours after dosing: re-exploring the therapeutic window. Eur Urol, 2004. 46: 357.
- Giuliano, F., et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract, 2010. 64: 240.
- Tsertsvadze, A., et al. Oral sildenafil citrate (viagra) for erectile dysfunction: a systematic review and metaanalysis of harms. Urology, 2009. 74: 831.
- Goldstein, I., et al. Oral sildenafil in the treatment of erectile dysfunction. 1998. J Urol, 2002. 167: 1197.
- Goldstein, I., et al. Efficacy and Safety of Sildenafil by Age in Men With Erectile Dysfunction. J Sex Med, 2016. 13: 852.
- Curran, M., et al. Tadalafil. Drugs, 2003. 63: 2203.
- Bella, A.J., et al. Tadalafil in the treatment of erectile dysfunction. Curr Urol Rep, 2003. 4: 472.
- Ventimiglia, E., et al. The safety of phosphodiesterase type 5 inhibitors for erectile dysfunction. Expert Opin Drug Saf, 2016. 15: 141.
- Chen, L., et al. Phosphodiesterase 5 inhibitors for the treatment of erectile dysfunction: A trade-off network meta-analysis. Eur Urol, 2015. 68: 674.
- Zhou, Z., et al. Meta-Analysis of the Long-Term Efficacy and Tolerance of Tadalafil Daily Compared With Tadalafil On-Demand in Treating Men With Erectile Dysfunction. Sexual medicine, 2019. 7: 282.
- Paduch, D.A., et al. Effects of 12 weeks of tadalafil treatment on ejaculatory and orgasmic dysfunction and sexual satisfaction in patients with mild to severe erectile dysfunction: integrated analysis of 17 placebocontrolled studies. BJU Int, 2013. 111: 334.
- Wang, Y., et al. Tadalafil 5 mg Once Daily Improves Lower Urinary Tract Symptoms and Erectile Dysfunction: A Systematic Review and Meta-analysis. Low Urin Tract Symptoms, 2018. 10: 84.
- Gacci, M., et al. Latest Evidence on the Use of Phosphodiesterase Type 5 Inhibitors for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Eur Urol, 2016. 70: 124.
- Roehrborn, C.G., et al. Erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH) combined responders to tadalafil after 12 weeks of treatment. BJU Int, 2016. 118: 153.
- Keating, G.M., et al. Vardenafil: a review of its use in erectile dysfunction. Drugs, 2003. 63: 2673.
- Capogrosso, P., et al. Time of onset of vardenafil orodispersible tablet in a real-life setting – looking beyond randomized clinical trials. Expert Rev Clin Pharmacol, 2017. 10: 339.
- Chung, E., et al. A state of art review on vardenafil in men with erectile dysfunction and associated underlying diseases. Expert Opin Pharmacother, 2011. 12: 1341.
- Sanford, M. Vardenafil orodispersible tablet. Drugs, 2012. 72: 87.
- Debruyne, F.M., et al. Time to onset of action of vardenafil: a retrospective analysis of the pivotal trials for the orodispersible and film-coated tablet formulations. J Sex Med, 2011. 8: 2912.
- Gittelman, M., et al. The POTENT II randomised trial: efficacy and safety of an orodispersible vardenafil formulation for the treatment of erectile dysfunction. Int J Clin Pract, 2010. 64: 594.
- Sperling, H., et al. The POTENT I randomized trial: efficacy and safety of an orodispersible vardenafil formulation for the treatment of erectile dysfunction. J Sex Med, 2010. 7: 1497.
- Wang, R., et al. Selectivity of avanafil, a PDE5 inhibitor for the treatment of erectile dysfunction: implications for clinical safety and improved tolerability. J Sex Med, 2012. 9: 2122.
- Kyle, J.A., et al. Avanafil for erectile dysfunction. Ann Pharmacother, 2013. 47: 1312.
- Goldstein, I., et al. A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J Sex Med, 2012. 9: 1122.
- Hellstrom, W.J., et al. Efficacy of Avanafil 15 Minutes after Dosing in Men with Erectile Dysfunction: A Randomized, Double-Blind, Placebo Controlled Study. J Urol, 2015.
- Wang, H., et al. The effectiveness and safety of avanafil for erectile dysfunction: a systematic review and metaanalysis. Curr Med Res Opin, 2014. 30: 1565.
- Corona, G., et al. The safety and efficacy of Avanafil, a new 2<sup>nd</sup> generation PDE5i: Comprehensive review and meta-analysis. Expert Opinion on Drug Safety, 2016. 15: 237.
- Mulhall, J.P., et al. A phase 3, placebo-controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. J Urol, 2013. 189: 2229.
- Madeira, C.R., et al. Efficacy and safety of oral phosphodiesterase 5 inhibitors for erectile dysfunction: a network meta-analysis and multicriteria decision analysis. World J Urol, 2021. 39: 953.
- Burns, P.R., et al. Treatment satisfaction of men and partners following switch from on-demand phosphodiesterase type 5 inhibitor therapy to tadalafil 5mg once daily. J Sex Med, 2015. 12: 720.
- Behr-Roussel, D., et al. Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: lack of tachyphylaxis. Eur Urol, 2005. 47: 87.
- Ferrini, M.G., et al. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology, 2006. 68: 429.
- Ferrini, M.G., et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod, 2007. 76: 915.
- Kovanecz, I., et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int, 2008. 101: 203.
- Vignozzi, L., et al. Effect of chronic tadalafil administration on penile hypoxia induced by cavernous neurotomy in the rat. J Sex Med, 2006. 3: 419.
- Brock, G., et al. Efficacy of Continuous Dosing of Tadalafil Once Daily vs Tadalafil On Demand in Clinical Subgroups of Men With Erectile Dysfunction: A Descriptive Comparison Using the Integrated Tadalafil Databases. J Sex Med, 2016. 13: 860.
- Porst, H., et al. Tadalafil once daily in men with erectile dysfunction: an integrated analysis of data obtained from 1913 patients from six randomized, double-blind, placebo-controlled, clinical studies. Eur Urol, 2014. 65: 455.
- Buvat, J., et al. Continuation and effectiveness of tadalafil once daily during a 6-month observational study in erectile dysfunction: the EDATE study. Int J Clin Pract, 2014. 68: 1087.
- Kloner, R.A., et al. Cardiovascular Safety of Phosphodiesterase Type 5 Inhibitors After Nearly 2 Decades on the Market. Sex Med Rev, 2018. 6: 583.
- Swearingen, D., et al. Hemodynamic effect of avanafil and glyceryl trinitrate coadministration. Drugs Context, 2013. 2013: 212248.
- Gur, S., et al. Update on drug interactions with phosphodiesterase-5 inhibitors prescribed as first-line therapy for patients with erectile dysfunction or pulmonary hypertension. Curr Drug Metab, 2013. 14: 265.
- Corona, G., et al. The use of phosphodiesterase 5 inhibitors with concomitant medications. J Endocrinol Invest, 2008. 31: 799.
- Kloner, R.A. Novel phosphodiesterase type 5 inhibitors: assessing hemodynamic effects and safety parameters. Clin Cardiol, 2004. 27: I20.
- Pickering, T.G., et al. Sildenafil citrate for erectile dysfunction in men receiving multiple antihypertensive agents: a randomized controlled trial. Am J Hypertens, 2004. 17: 1135.
- Satake, N., et al. Potentiating effect of nicorandil, an antianginal agent, on relaxation induced by isoproterenol in isolated rat aorta: involvement of cyclic GMP-inhibitable cyclic AMP phosphodiesterase. J Cardiovasc Pharmacol, 1995. 25: 489.
- Adamou, C., et al. The hemodynamic interactions of combination therapy with α-blockers and phosphodiesterase-5 inhibitors compared to monotherapy with α-blockers: a systematic review and metaanalysis. Int Urol Nephrol, 2020. 52: 1407.
- McCullough, A.R., et al. Achieving treatment optimization with sildenafil citrate (Viagra) in patients with erectile dysfunction. Urology, 2002. 60: 28.
- Hatzichristou, D., et al. Sildenafil failures may be due to inadequate patient instructions and follow-up: a study on 100 non-responders. Eur Urol, 2005. 47: 518.
- Forgue, S.T., et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol, 2006. 61: 280.
- Nichols, D.J., et al. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol, 2002. 53 Suppl 1: 5S.
- Rosen, R.C., et al. Determining the earliest time within 30 minutes to erectogenic effect after tadalafil 10 and 20 mg: a multicenter, randomized, double-blind, placebo-controlled, at-home study. J Sex Med, 2004. 1: 193.
- Montorsi, F., et al. Earliest time to onset of action leading to successful intercourse with vardenafil determined in an at-home setting: a randomized, double-blind, placebo-controlled trial. J Sex Med, 2004. 1: 168.
- Padma-Nathan, H., et al. Minimal time to successful intercourse after sildenafil citrate: results of a randomized, double-blind, placebo-controlled trial. Urology, 2003. 62: 400.
- Rajagopalan, P., et al. Effect of high-fat breakfast and moderate-fat evening meal on the pharmacokinetics of vardenafil, an oral phosphodiesterase-5 inhibitor for the treatment of erectile dysfunction. J Clin Pharmacol, 2003. 43: 260.
- Gruenwald, I., et al. Positive effect of counseling and dose adjustment in patients with erectile dysfunction who failed treatment with sildenafil. Eur Urol, 2006. 50: 134.
- Hatzimouratidis, K., et al. Treatment strategy for “non-responders” to tadalafil and vardenafil: a real-life study. Eur Urol, 2006. 50: 126.
- Park, N.C., et al. Treatment Strategy for Non-Responders to PDE5 Inhibitors. World J Mens Health, 2013. 31: 31.
- Porst, H., et al. SOP conservative (medical and mechanical) treatment of erectile dysfunction. J Sex Med, 2013. 10: 130.
- Mostafa, T., et al. Effect of Genetic Polymorphism on the Response to PDE5 Inhibitors in Patients With Erectile Dysfunction: A Systematic Review and a Critical Appraisal. Sex Med Rev, 2020. 8: 573.
- Marchal Escalona, C., et al. PDE5A Polymorphisms Influence on Sildenafil Treatment Success. J Sex Med, 2016. 13: 1104.
- Azevedo, A.M.M., et al. Relationship between asymmetric dimethylarginine, nitrite and genetic polymorphisms: Impact on erectile dysfunction therapy. Nitric Oxide, 2017. 71: 44.
- Lacchini, R., et al. Influence of arginase polymorphisms and arginase levels/activity on the response to erectile dysfunction therapy with sildenafil. Pharmacogenomics J, 2018. 18: 238.
- Mulhall, J.P., et al. The 2018 Revision to the Process of Care Model for Management of Erectile Dysfunction. J Sex Med, 2018. 15: 1434.
- Corona, G., et al. First-generation phosphodiesterase type 5 inhibitors dropout: a comprehensive review and meta-analysis. Andrology, 2016. 4: 1002.
- Corona, G., et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med, 2014. 11: 1577.
- Salonia, A., et al. Paediatric and adult-onset male hypogonadism. Nat Rev Dis Primers, 2019. 5: 38.
- Eardley, I., et al. Factors associated with preference for sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy: post hoc analysis of data from a multicentre, randomized, open-label, crossover study. BJU Int, 2007. 100: 122.
- Hatzimouratidis, K., et al. Psychosocial outcomes after initial treatment of erectile dysfunction with tadalafil once daily, tadalafil on demand or sildenafil citrate on demand: results from a randomized, open-label study. Int J Impot Res, 2014. 26: 223.
- Liao, X., et al. Comparative efficacy and safety of phosphodiesterase type 5 inhibitors for erectile dysfunction in diabetic men: a Bayesian network meta-analysis of randomized controlled trials. World J Urol, 2019. 37: 1061.
- Mykoniatis, I., et al. Assessment of Combination Therapies vs Monotherapy for Erectile Dysfunction: A Systematic Review and Meta-analysis. JAMA Netw Open, 2021. 4: e2036337.
- Cui, H., et al. Efficacy and safety of long-term tadalafil 5 mg once daily combined with sildenafil 50 mg as needed at the early stage of treatment for patients with erectile dysfunction. Andrologia, 2015. 47: 20.
- Anaissie, J., et al. Clinical use of alprostadil topical cream in patients with erectile dysfunction: a review. Res Rep Urol, 2016. 8: 123.
- Rooney, M., et al. Long-term, multicenter study of the safety and efficacy of topical alprostadil cream in male patients with erectile dysfunction. J Sex Med, 2009. 6: 520.
- Padma-Nathan, H., et al. An integrated analysis of alprostadil topical cream for the treatment of erectile dysfunction in 1732 patients. Urology, 2006. 68: 386.
- Cai, T., et al. The intra-meatal application of alprostadil cream (Vitaros®) improves drug efficacy and patient’s satisfaction: results from a randomized, two-administration route, cross-over clinical trial. Int J Impot Res, 2019. 31: 119.
- Padma-Nathan, H., et al. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med, 1997. 336: 1.
- Costa, P., et al. Intraurethral alprostadil for erectile dysfunction: a review of the literature. Drugs, 2012. 72: 2243.
- Mulhall, J.P., et al. Analysis of the consistency of intraurethral prostaglandin E(1) (MUSE) during at-home use. Urology, 2001. 58: 262.
- Shabsigh, R., et al. Intracavernous alprostadil alfadex is more efficacious, better tolerated, and preferred over intraurethral alprostadil plus optional actis: a comparative, randomized, crossover, multicenter study. Urology, 2000. 55: 109.
- Chung, E., et al. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: an Australian first open-label single-arm prospective clinical trial. BJU international, 2015. 5: 46.
- Gruenwald, I., et al. Shockwave treatment of erectile dysfunction. Ther Adv Urol, 2013. 5: 95.
- Gruenwald, I., et al. Low-intensity extracorporeal shock wave therapy–a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med, 2012. 9: 259.
- Olsen, A.B., et al. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scandinavian J Urol, 2015. 49: 329.
- Vardi, Y., et al. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month followup pilot study in patients with organic erectile dysfunction. Eur Urol, 2010. 58: 243.
- Kitrey, N.D., et al. Penile Low Intensity Shock Wave Treatment is Able to Shift PDE5i Nonresponders to Responders: A Double-Blind, Sham Controlled Study. J Urol, 2016. 195: 1550.
- Hisasue, S., et al. Impact of aging and comorbidity on the efficacy of low-intensity shock wave therapy for erectile dysfunction. Int J Urol, 2016. 23: 80.
- Fode, M., et al. Low-intensity shockwave therapy for erectile dysfunction: is the evidence strong enough? Nat Rev Urol, 2017. 14: 593.
- Sokolakis, I., et al. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: a systematic review and meta-analysis of randomised controlled trials. Int J Impot Res, 2019. 31: 177.
- Porst, H. Review of the Current Status of Low Intensity Extracorporeal Shockwave Therapy (Li-ESWT) in Erectile Dysfunction (ED), Peyronie’s Disease (PD), and Sexual Rehabilitation After Radical Prostatectomy With Special Focus on Technical Aspects of the Different Marketed ESWT Devices Including Personal Experiences in 350 Patients. Sex Med Rev, 2021. 9: 93.
- Kalyvianakis, D., et al. Low-intensity shockwave therapy (LiST) for erectile dysfunction: a randomized clinical trial assessing the impact of energy flux density (EFD) and frequency of sessions. Int J Impot Res, 2020. 32: 329.
- Kalyvianakis, D., et al. Low-Intensity Shockwave Therapy Improves Hemodynamic Parameters in Patients With Vasculogenic Erectile Dysfunction: A Triplex Ultrasonography-Based Sham-Controlled Trial. J Sex Med, 2017. 14: 891.
- Bechara, A., et al. Twelve-Month Efficacy and Safety of Low-Intensity Shockwave Therapy for Erectile Dysfunction in Patients Who Do Not Respond to Phosphodiesterase Type 5 Inhibitors. Sex Med, 2016. 4: e225.
- Vinay, J., et al. Penile low intensity shock wave treatment for PDE5I refractory erectile dysfunction: a randomized double-blind sham-controlled clinical trial. World J Urol, 2020.
- Chung, E., et al. Evaluation of Long-Term Clinical Outcomes and Patient Satisfaction Rate Following Low Intensity Shock Wave Therapy in Men With Erectile Dysfunction: A Minimum 5-Year Follow-Up on a Prospective Open-Label Single-Arm Clinical Study. Sex Med, 2021. 9: 100384.
- Baccaglini, W., et al. The Role of the Low-Intensity Extracorporeal Shockwave Therapy on Penile Rehabilitation After Radical Prostatectomy: A Randomized Clinical Trial. J Sex Med, 2020. 17: 688.
- Ladegaard, P.B.J., et al. Erectile Dysfunction A Prospective Randomized Placebo-Controlled Study Evaluating the Effect of Low-Intensity Extracorporeal Shockwave Therapy (LI-ESWT) in Men With Erectile Dysfunction Following Radical Prostatectomy. Sex Med, 2021. 9: 100338.
- Fojecki, G.L., et al. Effect of Low-Energy Linear Shockwave Therapy on Erectile Dysfunction-A Double-Blinded, Sham-Controlled, Randomized Clinical Trial. J Sex Med, 2017. 14: 106.
- Campbell, J.D., et al. Meta-analysis of randomized controlled trials that assess the efficacy of low-intensity shockwave therapy for the treatment of erectile dysfunction. Ther Adv Urol, 2019. 11: 1756287219838364.
- Dewitte, M., et al. A Psychosocial Approach to Erectile Dysfunction: Position Statements from the European Society of Sexual Medicine (ESSM). Sexual Medicine, 2021. 9: 100434.
- Bossio, J.A., et al. Mindfulness-Based Group Therapy for Men With Situational Erectile Dysfunction: A MixedMethods Feasibility Analysis and Pilot Study. J Sex Med, 2018. 15: 1478.
- Tajar, A., et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab, 2012. 97: 1508.
- Rizk, P.J., et al. Testosterone therapy improves erectile function and libido in hypogonadal men. Curr Opin Urol, 2017. 27: 511.
- Levine, L.A., et al. Vacuum constriction and external erection devices in erectile dysfunction. Urol Clin North Am, 2001. 28: 335.
- Yuan, J., et al. Vacuum therapy in erectile dysfunction–science and clinical evidence. Int J Impot Res, 2010. 22: 211.
- Cookson, M.S., et al. Long-term results with vacuum constriction device. J Urol, 1993. 149: 290.
- Lewis, R.W., et al. External vacuum therapy for erectile dysfunction: use and results. World J Urol, 1997. 15: 78.
- Trost, L.W., et al. External Mechanical Devices and Vascular Surgery for Erectile Dysfunction. J Sex Med, 2016. 13: 1579.
- Pajovic, B., et al. Vacuum erection device in treatment of organic erectile dysfunction and penile vascular differences between patients with DM type I and DM type II. Aging Male, 2017. 20: 49.
- Eardley, I., et al. Pharmacotherapy for erectile dysfunction. J Sex Med, 2010. 7: 524.
- Coombs, P.G., et al. A review of outcomes of an intracavernosal injection therapy programme. BJU Int, 2012. 110: 1787.
- Kattan, S., et al. Double-blind, cross-over study comparing prostaglandin E1 and papaverine in patients with vasculogenic impotence. Urology, 1991. 37: 516.
- Lakin, M.M., et al. Intracavernous injection therapy: analysis of results and complications. J Urol, 1990. 143: 1138.
- Moriel, E.Z., et al. Sodium bicarbonate alleviates penile pain induced by intracavernous injections for erectile dysfunction. J Urol, 1993. 149: 1299.
- Gupta, R., et al. Predictors of success and risk factors for attrition in the use of intracavernous injection. J Urol, 1997. 157: 1681.
- Sundaram, C.P., et al. Long-term follow-up of patients receiving injection therapy for erectile dysfunction. Urology, 1997. 49: 932.
- Seyam, R., et al. A prospective randomized study to optimize the dosage of trimix ingredients and compare its efficacy and safety with prostaglandin E1. Int J Impot Res, 2005. 17: 346.
- Vardi, Y., et al. Logistic regression and survival analysis of 450 impotent patients treated with injection therapy: long-term dropout parameters. J Urol, 2000. 163: 467.
- Porst, H., et al. Intracavernous Alprostadil Alfadex–an effective and well tolerated treatment for erectile dysfunction. Results of a long-term European study. Int J Impot Res, 1998. 10: 225.
- Duncan, C., et al. Erectile dysfunction: a global review of intracavernosal injectables. World J Urol, 2019. 37: 1007.
- Buvat, J., et al. Double-blind multicenter study comparing alprostadil alpha-cyclodextrin with moxisylyte chlorhydrate in patients with chronic erectile dysfunction. J Urol, 1998. 159: 116.
- Mulhall, J.P., et al. Intracavernosal forskolin: role in management of vasculogenic impotence resistant to standard 3-agent pharmacotherapy. J Urol, 1997. 158: 1752.
- Bechara, A., et al. Comparative study of papaverine plus phentolamine versus prostaglandin E1 in erectile dysfunction. J Urol, 1997. 157: 2132.
- McMahon CG, et al. A comparison of the response to the intracavernosal injection of papaverine and phentolamine, prostaglandin E1 and a combination of all three agents in the management of impotence. J Urol, 1999. 162.
- Dinsmore, W.W., et al. Vasoactive intestinal polypeptide/phentolamine for intracavernosal injection in erectile dysfunction. BJU Int, 2008. 102: 933.
- McMahon, C.G., et al. Treatment of intracorporeal injection nonresponse with sildenafil alone or in combination with triple agent intracorporeal injection therapy. J Urol, 1999. 162: 1992.
- Towe, M., et al. The use of combination regenerative therapies for erectile dysfunction: rationale and current status. Int J Impot Res, 2021.
- Epifanova M, et al. Combined therapy for treating erectile dysfunction: First results on the use of low intensity extracorporeal shock wave therapy and platelet-rich plasma. BJU Int, 2019. 123: (Suppl. 3) 25.
- Banno JJ, et al. The efficacy of platelet-rich plasma (PRP) as a supplemental therapy for the treatment of erectile dysfunction (ED): Initial outcomes. J Sex Med, 2017. 14.
- Chalyj, M.E., et al. [The effectiveness of intracavernous autologous platelet-rich plasma in the treatment of erectile dysfunction]. Urologiia, 2015: 76.
- Ruffo, A., et al. Effectiveness and safety of Platelet rich Plasma (PrP) cavernosal injections plus external shock wave treatment for penile erectile dysfunction: First results from a prospective, randomized, controlled, interventional study. Eur Urol Supplements, 2019. 18: e1622.
- Matz, E.L., et al. Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol, 2018. 59: 61.
- Alkhayal, S., et al. PO-01-091 Platelet rich plasma penile rejuvenation as a treatment for erectile dysfunction: An update. J Sex Med, 2019. 16: S71.
- Poulios, E., et al. Platelet-Rich Plasma (PRP) Improves Erectile Function: A Double-Blind, Randomized, PlaceboControlled Clinical Trial. J Sex Med, 2021. 18: 926.
- Oudelaar, B.W., et al. Concentrations of Blood Components in Commercial Platelet-Rich Plasma Separation Systems: A Review of the Literature. Am J Sports Med, 2019. 47: 479.
- Alkandari, M.H., et al. Platelet-Rich Plasma Injections for Erectile Dysfunction and Peyronie’s Disease: A Systematic Review of Evidence. Sex Med Rev, 2021.
- Mazzucco, L., et al. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang, 2009. 97: 110.
- Lee, H.W., et al. Ginseng for Erectile Dysfunction: A Cochrane Systematic Review. World J Mens Health, 2021.
- Xu, J., et al. Association between folic acid, homocysteine, vitamin B12 and erectile dysfunction-A crosssectional study. Andrologia, 2021. 53: e14234.
- Jo, J.K., et al. Effect of Starting Penile Rehabilitation with Sildenafil Immediately after Robot-Assisted Laparoscopic Radical Prostatectomy on Erectile Function Recovery: A Prospective Randomized Trial. J Urol, 2018. 199: 1600.
- Montorsi, F., et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol, 2008. 54: 924.
- Montorsi, F., et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT). Eur Urol, 2014. 65: 587.
- Montorsi, F., et al. Exploratory Decision-Tree Modeling of Data from the Randomized REACTT Trial of Tadalafil Versus Placebo to Predict Recovery of Erectile Function After Bilateral Nerve-Sparing Radical Prostatectomy. Eur Urol, 2016. 70: 529.
- Montorsi, F., et al. Efficacy of sildenafil citrate in men with erectile dysfunction following radical prostatectomy: a systematic review of clinical data. J Sex Med, 2005. 2: 658.
- Schwartz, E.J., et al. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol, 2004. 171: 771.
- Padma-Nathan, H., et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res, 2008. 20: 479.
- Kim, D.J., et al. A prospective, randomized, placebo-controlled trial of on-Demand vs. nightly sildenafil citrate as assessed by Rigiscan and the international index of erectile function. Andrology, 2016. 4: 27.
- Montorsi, F., et al. Tadalafil in the treatment of erectile dysfunction following bilateral nerve sparing radical retropubic prostatectomy: a randomized, double-blind, placebo-controlled trial. J Urol, 2004. 172: 1036.
- Patel, H.R., et al. Effects of tadalafil treatment after bilateral nerve-sparing radical prostatectomy: quality of life, psychosocial outcomes, and treatment satisfaction results from a randomized, placebo-controlled phase IV study. BMC Urol, 2015. 15: 31.
- Brock, G., et al. Safety and efficacy of vardenafil for the treatment of men with erectile dysfunction after radical retropubic prostatectomy. J Urol, 2003. 170: 1278.
- Nehra, A., et al. Vardenafil improved patient satisfaction with erectile hardness, orgasmic function and sexual experience in men with erectile dysfunction following nerve sparing radical prostatectomy. J Urol, 2005. 173: 2067.
- Philippou, Y.A., et al. Penile rehabilitation for postprostatectomy erectile dysfunction. Cochrane Database Syst Rev, 2018. 10: Cd012414.
- Montorsi, F., et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol, 1997. 158: 1408.
- Raina, R., et al. The early use of transurethral alprostadil after radical prostatectomy potentially facilitates an earlier return of erectile function and successful sexual activity. BJU Int, 2007. 100: 1317.
- Raina, R., et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res, 2006. 18: 77.
- Nason, G.J., et al. Efficacy of vacuum erectile devices (VEDs) after radical prostatectomy: the initial Irish experience of a dedicated VED clinic. Int J Impot Res, 2016. 28: 205.
- Hellstrom, W.J., et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med, 2010. 7: 501.
- Tal, R., et al. Penile implant utilization following treatment for prostate cancer: analysis of the SEER-Medicare database. J Sex Med, 2011. 8: 1797.
- Sridhar, A.N., et al. Recovery of Baseline Erectile Function in Men Following Radical Prostatectomy for High-Risk Prostate Cancer: A Prospective Analysis Using Validated Measures. J Sex Med, 2016. 13: 435.
- Qin, F., et al. The Early Use of Vacuum Therapy for Penile Rehabilitation After Radical Prostatectomy: Systematic Review and Meta-Analysis. Am J Mens Health, 2018. 12: 2136.
- Sari Motlagh, R., et al. Penile Rehabilitation Strategy after Nerve Sparing Radical Prostatectomy: A Systematic Review and Network Meta-Analysis of Randomized Trials. J Urol, 2021. 205: 1018.
- Feng, D., et al. Generating comprehensive comparative evidence on various interventions for penile rehabilitation in patients with erectile dysfunction after radical prostatectomy: a systematic review and network meta-analysis. Transl Androl Urol, 2021. 10: 109.
- Wong, C., et al. A Systematic Review of Pelvic Floor Muscle Training for Erectile Dysfunction After Prostatectomy and Recommendations to Guide Further Research. J Sex Med, 2020. 17: 737.
- Sohn, M., et al. Standard operating procedures for vascular surgery in erectile dysfunction: revascularization and venous procedures. J Sex Med, 2013. 10: 172.
- Antonini, G., et al. Minimally invasive infrapubic inflatable penile prosthesis implant for erectile dysfunction: Evaluation of efficacy, satisfaction profile and complications. Int J Impot Res. 2016. 28: 4.
- Bajic, P., et al. Etiology of Erectile Dysfunction and Duration of Symptoms in Patients Undergoing Penile Prosthesis: A Systematic Review. Sex Med Rev, 2020. 8: 333.
- Muneer, A., et al. UK practice for penile prosthesis surgery: baseline analysis of the British Association of Urological Surgeons (BAUS) Penile Prosthesis Audit. BJU Int, 2020.
- Martinez-Salamanca, J.I., et al. Penile prosthesis surgery in patients with corporal fibrosis: a state of the art review. J Sex Med, 2011. 8: 1880.
- Montague, D.K. Penile prosthesis implantation in the era of medical treatment for erectile dysfunction. Urol Clin North Am, 2011. 38: 217.
- Casabe, A.R., et al. Satisfaction assessment with malleable prosthetic implant of Spectra (AMS) and Genesis (Coloplast) models. Int J Impot Res, 2016. 28: 228.
- Mulcahy, J.J., et al. The penile implant for erectile dysfunction. J Sex Med, 2004. 1: 98.
- Montague, D.K., et al. Penile prosthesis implantation. Urol Clin North Am, 2001. 28: 355.
- Palmisano, F., et al. Comparison of Infrapubic vs Penoscrotal Approaches for 3-Piece Inflatable Penile Prosthesis Placement: Do We Have a Winner? Sex Med Rev, 2018. 6: 631.
- Bettocchi, C., et al. Patient and partner satisfaction after AMS inflatable penile prosthesis implant. J Sex Med, 2010. 7: 304.
- Falcone, M., et al. Prospective analysis of the surgical outcomes and patients’ satisfaction rate after the AMS Spectra penile prosthesis implantation. Urology, 2013. 82: 373.
- Henry, G.D., et al. A survey of patients with inflatable penile prostheses: assessment of timing and frequency of intercourse and analysis of implant durability. J Sex Med, 2012. 9: 1715.
- Kim, D.S., et al. AMS 700CX/CXM inflatable penile prosthesis has high mechanical reliability at long-term followup. J Sex Med, 2010. 7: 2602.
- Lux, M., et al. Outcomes and satisfaction rates for the redesigned 2-piece penile prosthesis. J Urol, 2007. 177: 262.
- Natali, A., et al. Penile implantation in Europe: successes and complications with 253 implants in Italy and Germany. J Sex Med, 2008. 5: 1503.
- Otero, J.R., et al. Comparison of the patient and partner satisfaction with 700CX and Titan penile prostheses. Asian J Androl, 2017. 19: 321.
- Chierigo, F., et al. Long-Term Follow-Up After Penile Prosthesis Implantation-Survival and Quality of Life Outcomes. J Sex Med, 2019. 16: 1827.
- Lee, D., et al. Simultaneous penile prosthesis and male sling/artificial urinary sphincter. Asian J Androl, 2013. 15: 10.
- Lee, D., et al. Combination surgery for erectile dysfunction and male incontinence. Curr Urol Rep, 2011. 12: 461.
- Segal, R.L., et al. Combined inflatable penile prosthesis-artificial urinary sphincter implantation: no increased risk of adverse events compared to single or staged device implantation. J Urol, 2013. 190: 2183.
- Pisano, F., et al. The importance of psychosexual counselling in the re-establishment of organic and erotic functions after penile prosthesis implantation. Int J Impot Res, 2015. 27: 197.
- Carson, C.C., et al. Efficacy, safety and patient satisfaction outcomes of the AMS 700CX inflatable penile prosthesis: results of a long-term multicenter study. AMS 700CX Study Group. J Urol, 2000. 164: 376.
- Wilson, S.K., et al. Comparison of mechanical reliability of original and enhanced Mentor Alpha I penile prosthesis. J Urol, 1999. 162: 715.
- Mandava, S.H., et al. Infection retardant coated inflatable penile prostheses decrease the incidence of infection: a systematic review and meta-analysis. J Urol, 2012. 188: 1855.
- Trost, L.W., et al. Long-term outcomes of penile prostheses for the treatment of erectile dysfunction. Expert Rev Med Devices, 2013. 10: 353.
- Chung, E., et al. A Worldwide Survey on Peyronie’s Disease Surgical Practice Patterns Among Surgeons. The J Sex Med, 2018. 15: 568.
- Mahon, J., et al. Infectious Adverse Events Following the Placement of a Penile Prosthesis: A Systematic Review. Sexual medicine reviews, 2019: S2050.
- Carson, C.C., 3rd, et al. Long-term infection outcomes after original antibiotic impregnated inflatable penile prosthesis implants: up to 7.7 years of followup. J Urol, 2011. 185: 614.
- Darouiche, R.O., et al. North American consensus document on infection of penile prostheses. Urology, 2013. 82: 937.
- Serefoglu, E.C., et al. Long-term revision rate due to infection in hydrophilic-coated inflatable penile prostheses: 11-year follow-up. J Sex Med, 2012. 9: 2182.
- Zargaroff, S., et al. National trends in the treatment of penile prosthesis infections by explantation alone vs. immediate salvage and reimplantation. J Sex Med, 2014. 11: 1078.
- Pineda, M., et al. Penile Prosthesis Infections-A Review of Risk Factors, Prevention, and Treatment. Sex Med Rev, 2016. 4: 389.
- Dropkin, B.M., et al. Antibiotics and Inflatable Penile Prosthesis Insertion: A Literature Review. Sex Med Rev, 2021. 9: 174.
- Bode, L.G., et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med, 2010. 362: 9.
- Christodoulidou, M., et al. Infection of Penile Prostheses in Patients with Diabetes Mellitus. Surg Infect (Larchmt), 2016. 17: 2.
- Hatzimouratidis, K., et al. EAU guidelines on penile curvature. Eur Urol, 2012. 62: 543.
- Henry, G.D., et al. An outcomes analysis of over 200 revision surgeries for penile prosthesis implantation: a multicenter study. J Sex Med, 2012. 9: 309.
- Levine, L.A., et al. Standard operating procedures for Peyronie’s disease. J Sex Med, 2013. 10: 230.
- Lipsky, M.J., et al. Diabetes Is a Risk Factor for Inflatable Penile Prosthesis Infection: Analysis of a Large Statewide Database. Sex Med, 2019. 7: 35.
- Canguven, O., et al. Is Hba1c level of diabetic patients associated with penile prosthesis implantation infections? Aging Male, 2018: 1.
- Towe, M., et al. Impact of Antimicrobial Dipping Solutions on Postoperative Infection Rates in Patients With Diabetes Undergoing Primary Insertion of a Coloplast Titan Inflatable Penile Prosthesis. J Sex Med, 2020. 17: 2077.
- Cihan, A., et al. The relationship between premature ejaculation and hyperthyroidism. J Urol, 2009. 181: 1273.
- Mulcahy, J.J. Long-term experience with salvage of infected penile implants. J Urol, 2000. 163: 481.
- Gross, M.S., et al. The Malleable Implant Salvage Technique: Infection Outcomes after Mulcahy Salvage Procedure and Replacement of Infected Inflatable Penile Prosthesis with Malleable Prosthesis. J Urol, 2016. 195: 694.
- Habous, M., et al. Conservative Therapy is an Effective Option in Patients With Localized Infection After Penile Implant Surgery. J Sex Med, 2016. 13: 972.
- Levine, L.A., et al. Penile Prosthesis Surgery: Current Recommendations From the International Consultation on Sexual Medicine. J Sex Med, 2016. 13: 489.
- Scherzer, N.D., et al. Penile Prosthesis Complications: Planning, Prevention, and Decision Making. Sexual medicine reviews, 2019. 7: 349.
- Hebert, K., et al. Acute Post-Inflatable Penile Prosthesis Glans Ischemia: Review of Incidence, Pathophysiology, and Management Recommendations. The J Sex Med, 2019. 16: 1.
- Akakpo, W., et al. Critical Analysis of Satisfaction Assessment After Penile Prosthesis Surgery. Sex Med Rev, 2017. 5: 244.
- Atri, E., et al. A Comparison Between AMS 700 and Coloplast Titan: A Systematic Literature Review. Cureus, 2020. 12: e11350.
- Althof, S.E., et al. Contemporary Management of Disorders of Male Orgasm and Ejaculation. Urology, 2016. 93: 9.
- Gao, J., et al. The impact of intravaginal ejaculatory latency time and erectile function on anxiety and depression in the four types of premature ejaculation: A large cross-sectional study in a chinese population. J Sex Med, 2014. 11: 521.
- Kempeneers, P., et al. Sexual Cognitions, Trait Anxiety, Sexual Anxiety, and Distress in Men With Different Subtypes of Premature Ejaculation and in Their Partners. J Sex Marital Ther, 2018. 44: 319.
- Rajkumar, R.P., et al. The association of anxiety with the subtypes of premature ejaculation: A chart review. rim Care Companion CNS Disord, 2014. 16: 10.4088.
- Ventus, D., et al. No Evidence for Long-Term Causal Associations Between Symptoms of Premature Ejaculation and Symptoms of Anxiety, Depression, and Sexual Distress in a Large, Population-Based Longitudinal Sample. J Sex Res, 2017. 54: 264.
- Yang, Y., et al. Correlations and stratification analysis between premature ejaculation and psychological disorders. Andrologia, 2019: e13315.
- Wiggins, A., et al. The Penile Sensitivity Ratio: A Novel Application of Biothesiometry to Assess Changes in Penile Sensitivity. J Sex Med, 2019. 16: 447.
- Chen, X., et al. Penile sensory thresholds in subtypes of premature ejaculation: implications of comorbid erectile dysfunction. Asian J Androl, 2018. 20: 330.
- Guo, L., et al. Significance of penile hypersensitivity in premature ejaculation. Sci Rep, 2017. 7: 10441.
- Xia, J.D., et al. A reassessment of penile sensory pathways and effects of prilocaine-lidocaine cream in primary premature ejaculation. Int J Impot Res, 2014. 26: 186.
- Salonia, A., et al. Quantitative sensory testing of peripheral thresholds in patients with lifelong premature ejaculation: a case-controlled study. J Sex Med, 2009. 6: 1755.
- Xin, Z.C., et al. Somatosensory evoked potentials in patients with primary premature ejaculation. J Urol, 1997. 158: 451.
- Xin, Z.C., et al. Penile sensitivity in patients with primary premature ejaculation. J Urol, 1996. 156: 979.
- Khan, H.L., et al. Serotonin transporter (5-HTTLPR) genotypes and trinucleotide repeats of androgen receptor exert a combinatorial effect on hormonal milieu in patients with lifelong premature ejaculation. Andrology, 2018. 6: 916.
- Roaiah, M.F., et al. Study of the prevalence of 5 HT-2C receptor gene polymorphisms in Egyptian patients with lifelong premature ejaculation. Andrologia, 2018. 50.
- Janssen, P.K., et al. The 5-HT2C receptor gene Cys23Ser polymorphism influences the intravaginal ejaculation latency time in Dutch Caucasian men with lifelong premature ejaculation. Asian J Androl, 2014. 16: 607.
- Janssen, P.K., et al. The 5-HT(1)A receptor C(1019)G polymorphism influences the intravaginal ejaculation latency time in Dutch Caucasian men with lifelong premature ejaculation. Pharmacol Biochem Behav, 2014. 121: 184.
- Hsieh, J.T., et al. The activation of peripheral 5-HT1A receptors can inhibit seminal vesicle contraction: an in vivo animal study. Urology, 2011. 78: 376.
- Janssen, P.K., et al. Serotonin transporter promoter region (5-HTTLPR) polymorphism is associated with the intravaginal ejaculation latency time in Dutch men with lifelong premature ejaculation. J Sex Med, 2009. 6: 276.
- Waldinger, M.D., et al. Changing paradigms from a historical DSM-III and DSM-IV view toward an evidencebased definition of premature ejaculation. Part I–validity of DSM-IV-TR. J Sex Med, 2006. 3: 682.
- Waldinger, M.D., et al. Changing paradigms from a historical DSM-III and DSM-IV view toward an evidencebased definition of premature ejaculation. Part II–proposals for DSM-V and ICD-11. J Sex Med, 2006. 3: 693.
- Waldinger, M.D., et al. Method and design of drug treatment research of subjective premature ejaculation in men differs from that of lifelong premature ejaculation in males: proposal for a new objective measure (part 1). Int J Impot Res, 2019.
- Waldinger, M.D. The pathophysiology of lifelong premature ejaculation. Transl Androl Urol, 2016. 5: 424.
- Carani, C., et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab, 2005. 90: 6472.
- Corona, G., et al. Psycho-biological correlates of rapid ejaculation in patients attending an andrologic unit for sexual dysfunctions. Eur Urol, 2004. 46: 615.
- McMahon, C.G., et al. The pathophysiology of acquired premature ejaculation. Transl Androl Urol, 2016. 5: 434.
- Symonds, T., et al. Further evidence of the reliability and validity of the premature ejaculation diagnostic tool. Int J Impot Res, 2007. 19: 521.
- Verze, P., et al. Premature Ejaculation Among Italian Men: Prevalence and Clinical Correlates From an Observational, Non-Interventional, Cross-Sectional, Epidemiological Study (IPER). Sexual medicine, 2018. 6: 193.
- Sihotang, R.C., et al. Premature ejaculation in patients with lower urinary tract symptoms: a systematic review. Int J Impot Res, 2020.
- Richardson, D., et al. Premature ejaculation–does country of origin tell us anything about etiology? J Sex Med, 2005. 2: 508.
- Waldinger, M.D., et al. Familial occurrence of primary premature ejaculation. Psychiatr Genet, 1998. 8: 37.
- Janssen, P.K., et al. Measurement errors in polymerase chain reaction are a confounding factor for a correct interpretation of 5-HTTLPR polymorphism effects on lifelong premature ejaculation: a critical analysis of a previously published meta-analysis of six studies. PLoS One, 2014. 9: e88031.
- Jern, P., et al. A reassessment of the possible effects of the serotonin transporter gene linked polymorphism 5-HTTLPR on premature ejaculation. Arch Sex Behav, 2013. 42: 45.
- Jern, P., et al. Preliminary Evidence for an Association Between Variants of the Catechol-O-Methyltransferase (COMT) Gene and Premature Ejaculation. J Sex Med, 2017. 14: 1558.
- Screponi, E., et al. Prevalence of chronic prostatitis in men with premature ejaculation. Urology, 2001. 58: 198.
- Shamloul, R., et al. Chronic prostatitis in premature ejaculation: a cohort study in 153 men. J Sex Med, 2006. 3: 150.
- Chierigo, F., et al. Lower urinary tract symptoms and depressive symptoms among patients presenting for distressing early ejaculation. Int J Impot Res, 2019.
- Culha, M.G., et al. Frequency of etiological factors among patients with acquired premature ejaculation: prospective, observational, single-center study. Int J Impot Res, 2019: 10.1038/s41443.
- Corona, G., et al. Hypoprolactinemia: a new clinical syndrome in patients with sexual dysfunction. J Sex Med, 2009. 6: 1457.
- Corona, G., et al. Premature and delayed ejaculation: two ends of a single continuum influenced by hormonal milieu. Int J Androl, 2011. 34: 41.
- Kadihasanoglu, M., et al. Relation between blood vitamin B12 levels with premature ejaculation: case-control study. Andrologia, 2017. 49.
- Abd El Aal, A.M., et al. Serum vitamin D level may be a novel potential risk factor for premature ejaculation: a comparative study. Int Urol Nephrol, 2018. 50: 1975.
- Majzoub, A., et al. Premature ejaculation in type II diabetes mellitus patients: Association with glycemic control. Transl Androl Urol, 2016. 5: 248.
- Bellastella, G., et al. Premature ejaculation is associated with glycemic control in Type 1 diabetes. J Sex Med, 2015. 12: 93.
- Jeh, S.U., et al. Metabolic Syndrome Is an Independent Risk Factor for Acquired Premature Ejaculation. World J Mens Health, 2019. 37: 226.
- Bolat, D., et al. The relationship between acquired premature ejaculation and metabolic syndrome: a prospective, comparative study. Int J Impot Res, 2017. 29: 105.
- Ventus, D., et al. Lifestyle Factors and Premature Ejaculation: Are Physical Exercise, Alcohol Consumption, and Body Mass Index Associated With Premature Ejaculation and Comorbid Erectile Problems? J Sex Med, 2016. 13: 1482.
- Dunn, K.M., et al. Association of sexual problems with social, psychological, and physical problems in men and women: a cross sectional population survey. J Epidemiol Community Health, 1999. 53: 144.
- Xia, Y., et al. Relationship between premature ejaculation and depression: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore), 2016. 95: e4620.
- El-Nashaar, A., et al. Antibiotic treatment can delay ejaculation in patients with premature ejaculation and chronic bacterial prostatitis. J Sex Med, 2007. 4: 491.
- Rowland, D., et al. Self-reported premature ejaculation and aspects of sexual functioning and satisfaction. J Sex Med, 2004. 1: 225.
- Rowland, D.L., et al. The psychological burden of premature ejaculation. J Urol, 2007. 177: 1065.
- Hanafy, S., et al. Prevalence of premature ejaculation and its impact on the quality of life: Results from a sample of Egyptian patients. Andrologia, 2019: e13298.
- Abdo, C.H. The impact of ejaculatory dysfunction upon the sufferer and his partner. Transl Androl Urol, 2016. 5: 460.
- Burri, A., et al. Female partner’s perception of premature ejaculation and its impact on relationship breakups, relationship quality, and sexual satisfaction. J Sex Med, 2014. 11: 2243.
- Byers, E.S., et al. Premature or rapid ejaculation: heterosexual couples’ perceptions of men’s ejaculatory behavior. Arch Sex Behav, 2003. 32: 261.
- Canat, L., et al. The relationship between female sexual function index domains and premature ejaculation. Int Urol Nephrol, 2018. 50: 633.
- Limoncin, E., et al. Premature ejaculation results in female sexual distress: standardization and validation of a new diagnostic tool for sexual distress. J Urol, 2013. 189: 1830.
- Solursh, D.S., et al. The human sexuality education of physicians in North American medical schools. Int J Impot Res, 2003. 15 Suppl 5: S41.
- Sotomayor, M. The burden of premature ejaculation: the patient’s perspective. J Sex Med, 2005. 2 Suppl 2: 110.
- Parnham, A., et al. Classification and definition of premature ejaculation. Transl Androl Urol, 2016. 5: 416.
- W.H.O. International Classification of Diseases 11th Revision for Mortality and Morbidity Statistics (ICD- 11-MMS). The global standard for diagnostic health information. 2018.
- Serefoglu, E.C., et al. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second International Society for Sexual Medicine Ad Hoc Committee for the Definition of Premature Ejaculation. J Sex Med, 2014. 11: 1423.
- Waldinger, M.D., et al. Differences between ICD-11 MMS and DSM-5 definition of premature ejaculation: a continuation of historical inadequacies and a source of serious misinterpretation by some European Regulatory Agencies (PART 2). Int J Impot Res, 2019. 31: 310.
- Shabsigh, R. Diagnosing premature ejaculation: a review. J Sex Med, 2006. 3 Suppl 4: 318.
- Sharlip, I. Diagnosis and treatment of premature ejaculation: the physician’s perspective. J Sex Med, 2005. 2 Suppl 2: 103.
- Rowland, D.L., et al. Premature ejaculation: psychophysiological considerations in theory, research, and treatment. Annu Rev Sex Res, 1997. 8: 224.
- Althof, S.E. Prevalence, characteristics and implications of premature ejaculation/rapid ejaculation. J Urol, 2006. 175: 842.
- Althof, S.E., et al. Patient reported outcomes used in the assessment of premature ejaculation. Urol Clin North Am, 2007. 34: 581.
- Waldinger, M.D., et al. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res, 2004. 16: 369.
- Waldinger, M.D. Towards evidence-based drug treatment research on premature ejaculation: a critical evaluation of methodology. Int J Impot Res, 2003. 15: 309.
- Giuliano, F., et al. Premature ejaculation: results from a five-country European observational study. Eur Urol, 2008. 53: 1048.
- Patrick, D.L., et al. Premature ejaculation: an observational study of men and their partners. J Sex Med, 2005. 2: 358.
- Kempeneers, P., et al. Functional and psychological characteristics of belgian men with premature ejaculation and their partners. Arch Sex Behav, 2013. 42: 51.
- Rosen, R.C., et al. Correlates to the clinical diagnosis of premature ejaculation: results from a large observational study of men and their partners. J Urol, 2007. 177: 1059.
- Lee, W.K., et al. Can estimated intravaginal ejaculatory latency time be used interchangeably with stopwatchmeasured intravaginal ejaculatory latency time for the diagnosis of lifelong premature ejaculation? Urology, 2015. 85: 375.
- Althof, S.E. Psychosexual therapy for premature ejaculation. Transl Androl Urol, 2016. 5: 475.
- Cormio, L., et al. The Combination of Dapoxetine and Behavioral Treatment Provides Better Results than Dapoxetine Alone in the Management of Patients with Lifelong Premature Ejaculation. J Sex Med, 2015. 12: 1609.
- Pavone, C., et al. Premature ejaculation: Pharmacotherapy vs group psychotherapy alone or in combination. Arch Ital Urol Androl, 2017. 89: 114.
- Melnik, T., et al. Psychosocial interventions for premature ejaculation. Cochrane Database Syst Rev, 2011: Cd008195.
- Porst, H., et al. Baseline characteristics and treatment outcomes for men with acquired or lifelong premature ejaculation with mild or no erectile dysfunction: integrated analyses of two phase 3 dapoxetine trials. The J Sex Med, 2010. 7: 2231.
- EMA. Fortacin: Summary of product characteristics. 2014.
- Pryor, J.L., et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet, 2006. 368: 929.
- Qin, Z., et al. Safety and efficacy characteristics of oral drugs in patients with premature ejaculation: a Bayesian network meta-analysis of randomized controlled trials. Int J Impot Res, 2019.
- Jian, Z., et al. Pharmacotherapy of premature ejaculation: a systematic review and network meta-analysis. Int Urol Nephrol, 2018. 50: 1939.
- Sridharan, K., et al. Pharmacological interventions for premature ejaculation: a mixed-treatment comparison network meta-analysis of randomized clinical trials. Int J Impot Res, 2018. 30: 215.
- Castiglione, F., et al. Current Pharmacological Management of Premature Ejaculation: A Systematic Review and Meta-analysis. Eur Urol, 2016. 69: 904.
- Modi, N.B., et al. Single- and multiple-dose pharmacokinetics of dapoxetine hydrochloride, a novel agent for the treatment of premature ejaculation. J Clin Pharmacol, 2006. 46: 301.
- McMahon, C.G. Dapoxetine: a new option in the medical management of premature ejaculation. Ther Adv Urol, 2012. 4: 233.
- McMahon, C.G., et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: integrated analysis of results from five phase 3 trials. J Sex Med, 2011. 8: 524.
- Li, J., et al. Dapoxetine for the treatment of premature ejaculation: a meta-analysis of randomized controlled trials with trial sequential analysis. Ann Saudi Med, 2018. 38: 366.
- Morales, A., et al. A review of the current status of topical treatments for premature ejaculation. BJU Int, 2007. 100: 493.
- Sachs, B.D., et al. Maintenance of erection of penile glans, but not penile body, after transection of rat cavernous nerves. J Urol, 1991. 146: 900.
- Wieder, J.A., et al. Anesthetic block of the dorsal penile nerve inhibits vibratory-induced ejaculation in men with spinal cord injuries. Urology, 2000. 55: 915.
- James, M.M.S., et al. Topical anaesthetics for premature ejaculation: A systematic review and meta-analysis. Sexual Health, 2016. 13: 114.
- Pu, C., et al. Topical anesthetic agents for premature ejaculation: a systematic review and meta-analysis. Urology, 2013. 81: 799.
- Liu, H., et al. Comparative efficacy and safety of drug treatment for premature ejaculation: A systemic review and Bayesian network meta-analysis. Andrologia, 2020: e13806.
- Atikeler, M.K., et al. Optimum usage of prilocaine-lidocaine cream in premature ejaculation. Andrologia, 2002. 34: 356.
- Porst, H., et al. Fortacin Spray for the Treatment of Premature Ejaculation. Urologia, 2017. 84: 1.
- McMahon, C.G., et al. Efficacy of sildenafil citrate (Viagra) in men with premature ejaculation. J Sex Med, 2005. 2: 368.
- Abu El-Hamd, M. Efficacy and safety of daily use of tadalafil in treatment of patients with premature ejaculation: A randomised placebo-controlled clinical trial. Andrologia, 2018. 50: e13005.
- Salonia, A., et al. A prospective study comparing paroxetine alone versus paroxetine plus sildenafil in patients with premature ejaculation. J Urol, 2002. 168: 2486.
- Zhang, X.S., et al. [Comparison between sildenafil plus sertraline and sertraline alone in the treatment of premature ejaculation]. Zhonghua Nan Ke Xue, 2005. 11: 520.
- Chen, J., et al. Efficacy of sildenafil as adjuvant therapy to selective serotonin reuptake inhibitor in alleviating premature ejaculation. Urology, 2003. 61: 197.
- Abu El-Hamd, M., et al. Comparison of the clinical efficacy and safety of the on-demand use of paroxetine, dapoxetine, sildenafil and combined dapoxetine with sildenafil in treatment of patients with premature ejaculation: A randomised placebo-controlled clinical trial. Andrologia, 2018. 50.
- Polat, E.C., et al. Combination therapy with selective serotonin reuptake inhibitors and phosphodiesterase-5 inhibitors in the treatment of premature ejaculation. Andrologia, 2015. 47: 487.
- Tang, W., et al. [Clinical efficacy of Viagra with behavior therapy against premature ejaculation]. Zhonghua Nan Ke Xue, 2004. 10: 366.
- Bhat, G.S., et al. Effectiveness of ‘on demand’ silodosin in the treatment of premature ejaculation in patients dissatisfied with dapoxetine: a randomized control study. Cent European J Urol, 2016. 69: 280.
- Sato, Y., et al. Silodosin versus naftopidil in the treatment of premature ejaculation: A prospective multicenter trial. Int J Urol, 2017. 24: 626.
- Sato, Y., et al. Silodosin and its potential for treating premature ejaculation: a preliminary report. Int J Urol, 2012. 19: 268.
- Tuken, M., et al. On-demand Modafinil Improves Ejaculation Time and Patient-reported Outcomes in Men With Lifelong Premature Ejaculation. Urology, 2016. 94: 139.
- Sahin, S., et al. A Prospective Randomized Controlled Study to Compare Acupuncture and Dapoxetine for the Treatment of Premature Ejaculation. Urol Int, 2016. 97: 104.
- Kim, J.J., et al. Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation. Int J Impot Res, 2004. 16: 547.
- Kwak, T.I., et al. Long-term effects of glans penis augmentation using injectable hyaluronic acid gel for premature ejaculation. Int J Impot Res, 2008. 20: 425.
- Abdallah, H., et al. Treatment of premature ejaculation by glans penis augmentation using hyaluronic acid gel: a pilot study. Andrologia, 2012. 44 Suppl 1: 650.
- Alahwany, A., et al. Hyaluronic acid injection in glans penis for treatment of premature ejaculation: a randomized controlled cross-over study. Int J Impot Res, 2019.
- Ahn, S.T., et al. Complications of glans penis augmentation. Int J Impot Res, 2019. 31: 245.
- Clement, P., et al. Inhibition of ejaculation by the non-peptide oxytocin receptor antagonist GSK557296: a multilevel site of action. Br J Pharmacol, 2013. 169: 1477.
- Shinghal, R., et al. Safety and efficacy of epelsiban in the treatment of men with premature ejaculation: a randomized, double-blind, placebo-controlled, fixed-dose study. J Sex Med, 2013. 10: 2506.
- Osterloh, I.H., et al. Pharmacokinetics, Safety, and Tolerability of Single Oral Doses of a Novel Oxytocin Receptor Antagonist-Cligosiban-in Development for Premature Ejaculation: Three Randomized Clinical Trials in Healthy Subjects. J Sex Med, 2018. 15: 1547.
- Wayman, C., et al. Cligosiban, A Novel Brain-Penetrant, Selective Oxytocin Receptor Antagonist, Inhibits Ejaculatory Physiology in Rodents. J Sex Med, 2018. 15: 1698.
- McMahon, C., et al. The Oxytocin Antagonist Cligosiban Prolongs Intravaginal Ejaculatory Latency and Improves Patient-Reported Outcomes in Men with Lifelong Premature Ejaculation: Results of a Randomized, Double-Blind, Placebo-Controlled Proof-of-Concept Trial (PEPIX). J Sex Med, 2019. 16: 1178.
- Jiang, M., et al. The efficacy of regular penis-root masturbation, versus Kegel exercise in the treatment of primary premature ejaculation: A quasi-randomised controlled trial. Andrologia, 2020. 52: e13473.
- Ventus, D., et al. Vibrator-Assisted Start-Stop Exercises Improve Premature Ejaculation Symptoms: A Randomized Controlled Trial. Arch Sex Behav, 2020. 49: 1559.
- Shechter, A., et al. Transcutaneous functional electrical stimulation-a novel therapy for premature ejaculation: results of a proof of concept study. Int J Impot Res, 2020. 32: 440.
- Uribe, O.L., et al. Transcutaneous electric nerve stimulation to treat patients with premature ejaculation: phase II clinical trial. Int J Impot Res, 2020. 32: 434.
- Joshi, A.M., et al. Role of Yoga in the Management of Premature Ejaculation. World J Mens Health, 2020. 38: 495.
- Rowland, D.L., et al. Moving Toward Empirically Based Standardization in the Diagnosis of Delayed Ejaculation. J Sex Med, 2020. 17: 1896.
- Shin, D.H., et al. The Evaluation and Treatment of Delayed Ejaculation. Sexual Medicine Reviews, 2014. 2: 121.
- Abdel-Hamid, I.A., et al. Delayed Ejaculation: Pathophysiology, Diagnosis, and Treatment. World J Mens Health, 2018. 36: 22.
- Martin-Tuite, P., et al. Management Options for Premature Ejaculation and Delayed Ejaculation in Men. Sex Med Rev, 2020. 8: 473.
- Corona, G., et al. The hormonal control of ejaculation. Nat Rev Urol, 2012. 9: 508.
- Morgentaler, A., et al. Delayed Ejaculation and Associated Complaints: Relationship to Ejaculation Times and Serum Testosterone Levels. J Sex Med, 2017. 14: 1116.
- Paduch, D.A., et al. Clinical and Demographic Correlates of Ejaculatory Dysfunctions Other Than Premature Ejaculation: A Prospective, Observational Study. J Sex Med, 2015. 12: 2276.
- Butcher, M.J., et al., Treatment of Delayed Ejaculation, In: The Textbook of Clinical Sexual Medicine, W.W. IsHak, Editor. 2017, Springer International Publishing: Cham.
- Rowland, D., et al. Disorders of orgasm and ejaculation in men. J Sex Med, 2010. 7: 1668.
- Martin-Tuite, P., et al. Management Options for Premature Ejaculation and Delayed Ejaculation in Men. Sex Med Rev, 2020. 8: 473.
- Butcher, M.J., et al. How is delayed ejaculation defined and treated in North America? Andrology, 2015. 3: 626.
- Nelson, C.J., et al. Assessment of penile vibratory stimulation as a management strategy in men with secondary retarded orgasm. Urology, 2007. 69: 552.
- Soler, J.M., et al. Midodrine improves orgasm in spinal cord-injured men: the effects of autonomic stimulation. J Sex Med, 2008. 5: 2935.
- Geboes, K., et al. Primary anejaculation: diagnosis and therapy. Feril Steril, 1975. 26: 1018.
- Ohl, D.A., et al. Anejaculation and retrograde ejaculation. Urol Clin North Am, 2008. 35: 211.
- Brindley, G.S. Reflex ejaculation under vibratory stimulation in paraplegic men. Paraplegia, 1981. 19: 299.
- Schatte Edward, C., et al. Treatment of infertility due to anejaculation in the male with electroejaculation and intracytoplasmic sperm injection. J Urol, 2000. 163: 1717.
- Esteves, S.C., et al. An update on sperm retrieval techniques for azoospermic males. Clinics (Sao Paulo), 2013. 68 Suppl 1: 99.
- Maurer, C.A., et al. Total mesorectal excision preserves male genital function compared with conventional rectal cancer surgery. Br J Surg, 2001. 88: 1501.
- Parnham, A., et al. Retrograde ejaculation, painful ejaculation and hematospermia. Transl Androl Urol, 2016. 5: 592.
- Edwards, A. Chronic disease of the colliculus seminalis. Brit Med J, 1909. 2: 1672.
- Grosse, A.B. Remarks on Impotentia Cocundi and Sexual Neurasthenia and Their Treatment. California State J Med, 1911. 9: 25.
- Irwin, W.K. Pain in genito-urinary affections: Its Variations and their Interpretation. Brit Med J, 1922. 2: 457.
- Wagenlehner, F.M., et al. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. Eur Urol, 2013. 63: 953.
- Tran, C.N., et al. Sexual dysfunction in chronic prostatitis/chronic pelvic pain syndrome. World J Urol, 2013. 31: 741.
- Kleinberg, L., et al. Treatment-related symptoms during the first year following transperineal 125I prostate implantation. Int J Radiat Oncol Biol Phys, 1994. 28: 985.
- Walz, J., et al. Ejaculatory disorders may affect screening for prostate cancer. J Urol, 2007. 178: 232.
- Koeman, M., et al. Orgasm after radical prostatectomy. British J Urol, 1996. 77: 861.
- Matsushita, K., et al. The evolution of orgasmic pain (dysorgasmia) following radical prostatectomy. J Sex Med, 2012. 9: 1454.
- Merrick, G.S., et al. Short-term sexual function after prostate brachytherapy. Int J Cancer, 2001. 96: 313.
- Butler, J.D., et al. Painful ejaculation after inguinal hernia repair. J Royal Soc Med, 1998. 91: 432.
- Aizenberg, D., et al. Painful ejaculation associated with antidepressants in four patients. J Clin Psych, 1991. 52: 461.
- Kulik, F.A., et al. Case report of painful ejaculation as a side effect of amoxapine. Am J Psych, 1982. 139: 234.
- Michael, A. Venlafaxine-induced painful ejaculation. Br J Psychiatry, 2000. 177: 282.
- Lange, W.R., et al. Can ciguatera be a sexually transmitted disease? J Toxicol Clin Toxicol, 1989. 27: 193.
- Senthilkumaran, S., et al. Painful ejaculation. Something fishy. Saudi Med J, 2010. 31: 451.
- Demyttenaere, K., et al. Painful ejaculation and urinary hesitancy in association with antidepressant therapy: relief with tamsulosin. Eur Neuropsychopharmacol, 2002. 12: 337.
- Jordi, P., et al. Management of ejaculation pain with topiramate: a case report. Clin J Pain, 2004. 20: 368.
- Cornel, E.B., et al. The effect of biofeedback physical therapy in men with Chronic Pelvic Pain Syndrome Type III. Eur Urol, 2005. 47: 607.
- Tuhkanen, K., et al. Sexual function of LUTS patients before and after neodymium laser prostatectomy and transurethral resection of prostate. A prospective, randomized trial. Urologia Internationalis, 2004. 73: 137.
- Krause, W. Transurethral resection of the ejaculatory ducts for treating ejaculatory symptoms. BJU international, 2005. 96: 1145.
- Giuliano, F., et al. Physiology of ejaculation: emphasis on serotonergic control. Eur Urol, 2005. 48: 408.
- Proctor, K.G., et al. The effect of sympathomimetic drugs on post-lymphadenectomy aspermia. J Urol, 1983. 129: 837.
- Gilja, I., et al. Retrograde ejaculation and loss of emission: possibilities of conservative treatment. Eur Urol, 1994. 25: 226.
- Hotchkiss, R.S., et al. Artificial insemination with semen recovered from the bladder. Feril Steril, 1954. 6: 37.
- Templeton, A., et al. Successful circumvention of retrograde ejaculation in an infertile diabetic man. Case report. Br J Obstet Gynaecol, 1982. 89: 1064.
- Crich, J.P., et al. Infertility in men with retrograde ejaculation: the action of urine on sperm motility, and a simple method for achieving antegrade ejaculation. Feril Steril, 1978. 30: 572.
- Jenkins, L.C., et al. Delayed orgasm and anorgasmia. Feril Steril, 2015. 104: 1082.
- Calabrò, R.S., et al. Anorgasmia during pregabalin add-on therapy for partial seizures. Epileptic Disord, 2013. 15: 358.
- Althof, S.E. Psychological interventions for delayed ejaculation/orgasm. Int J Impot Res, 2012. 24: 131.
- McMahon, C.G., et al. Standard operating procedures in the disorders of orgasm and ejaculation. The J Sex Med, 2013. 10: 204.
- McCormick, S., et al. Reversal of fluoxetine-induced anorgasmia by cyproheptadine in two patients. J Clin Psych, 1990. 51: 383.
- Balon, R. Intermittent amantadine for fluoxetine-induced anorgasmia. J Sex Marital Ther, 1996. 22: 290.
- Balogh, S., et al. Treatment of fluoxetine-induced anorgasmia with amantadine. J Clin Psych, 1992. 53: 212.
- Price, J., et al. Treatment of clomipramine-induced anorgasmia with yohimbine: a case report. J Clin Psych, 1990. 51: 32.
- Jacobsen, F.M. Fluoxetine-induced sexual dysfunction and an open trial of yohimbine. J Clin Psych, 1992. 53: 119.
- Ashton, A.K., et al. Bupropion as an antidote for serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psych, 1998. 59: 112.
- Kumar, P., et al. Haematospermia – a systematic review. Ann R Coll Surg Engl, 2006. 88: 339.
- Ahmad, I., et al. Hemospermia. J Urol, 2007. 177: 1613.
- Efesoy, O., et al. Hematospermia is rarely related to genitourinary cancer: lessons learned from 15 years of experience with 342 cases. Int J Impot Res, 2020.
- Akhter, W., et al. Should every patient with hematospermia be investigated? A critical review. Central European J Urol, 2013. 66: 79.
- Hosseinzadeh, K., et al. ACR Appropriateness Criteria((R)) Hematospermia. J Am Coll Radiol, 2017. 14: S154.
- Bhaduri, S., et al. Haematospermia associated with malignant hypertension. Sexually Transmitted Infections, 1999. 75: 200.
- Close, C.F., et al. The association between haemospermia and severe hypertension. Postgrad Med J, 1991. 67: 157.
- Bamberger, E., et al. Detection of sexually transmitted pathogens in patients with hematospermia. Israel Med Ass J: IMAJ, 2005. 7: 224.
- Munkel witz, R., et al. Current perspectives on hematospermia: a review. J Androl, 1997. 18: 6.
- Cho, I.R., et al. Magnetic resonance imaging in hemospermia. J Urol, 1997. 157: 258.
- Lencioni, R., et al. Endorectal coil MR imaging findings in hemospermia. Magma (New York, N.Y.), 1999. 8: 91.
- Li, Y.-F., et al. Imaging diagnosis, transurethral endoscopic observation, and management of 43 cases of persistent and refractory hematospermia. J Androl, 2012. 33: 906.
- Cui, Z.-Q., et al. [Transurethral seminal vesiculoscopy combined with finasteride for recurrent hematospermia]. Zhonghua Nan Ke Xue, 2014. 20: 536.
- Liu, Z.-Y., et al. Transurethral seminal vesiculoscopy in the diagnosis and treatment of persistent or recurrent hemospermia: a single-institution experience. Asian J Androl, 2009. 11: 566.
- Xing, C., et al. Prospective trial comparing transrectal ultrasonography and transurethral seminal vesiculoscopy for persistent hematospermia. Int J Urol, 2012. 19: 437.
- Lowell, D.M., et al. Melanospermia: a hitherto undescribed entity. J Urol, 1966. 95: 407.
- Smith, G.W., et al. Melanospermia: an unusual presentation of malignant melanoma. J Urol, 1973. 110: 314.
- Manohar, T., et al. Transrectal ultrasound- and fluoroscopic-assisted transurethral incision of ejaculatory ducts: a problem-solving approach to nonmalignant hematospermia due to ejaculatory duct obstruction. J Endourol, 2008. 22: 1531.
- Fuse, H., et al. Transurethral incision for hematospermia caused by ejaculatory duct obstruction. Archives of Andrology, 2003. 49: 433.
- Mittal, P.K., et al. Hematospermia Evaluation at MR Imaging. Radiographics, 2016. 36: 1373.
- Suh, Y., et al. Etiologic classification, evaluation, and management of hematospermia. Transl Androl Urol, 2017. 6: 959.
Author Correspondence:
Prof. Dr. Semir A. Salim. Al Samarrai
Medical Director of Professor Al Samarrai Medical Center.
Dubai Healthcare City, Al-Razi Building 64, Block D, 2nd Floor, Suite 2018
E-mail: semiralsamarrai@hotmail.com
Tel: +97144233669
